Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Laconic Review on Capillary Electrophoresis-Mass Spectrometry in Separation of Proteins
Authors: Attish Bhardwaj, Aman Sharma, Upasana Thakur, Amar Deep Ankalgi
DOI Link: https://doi.org/10.22214/ijraset.2023.54567
Certificate: View Certificate
Abstract
The separation technique known as electrophoresis is made possible by the ability of liquid molecules to travel through an electric field. Early in the 1980s, it was discovered that considerable separation efficiencies could be obtained by passing a voltage across a capillary. In the late 1980s and early 1990s, commercial capillary electrophoresis (CE) first became accessible. Mass spectrometry is a powerful tool for identifying unknowns, studying molecular structure, and investigating the fundamental principles of chemistry. The most efficient technique combines high-resolution separations by CE with high-sensitivity and selectivity detections by MS, which is typical for bioanalytical applications and targets or thoroughly high throughput studies of low-concentration analytes frequently occurring in complicated matrices. The combination of capillary electrophoresis with mass spectrometry, or CE-MS, has emerged as an effective standard technique for the analysis of a wide variety of analytes. CE-MS, a proteomic technology that combines chromatographic techniques with MS to increase the resolving power, is a well-liked technique for proteome analysis.
Introduction
I. INTRODUCTION
The capacity of liquid molecules to move across an electric field allows for the separation process known as electrophoresis. The most popular technique for investigate biomolecules in molecular biology as well as in biochemistry, including genetic components like DNA or RNA, proteins, electrophoresis, in all of its varied forms or sorts. While submerged in a solution buffer, charged particles are separated using several types of electrophoresis. For all electrophoresis procedures, an electrophoresis unit, sometimes referred to as an electrophoresis chamber, is necessary. [1]
A. Overview of CE& MS
In the early 1980s, it became clear that applying a voltage across a capillary could provide significant separation efficiencies. Commercial capillary electrophoresis (CE) first became available in the late 1980s and early 1990s.The spectrum of separation mechanisms in CE and instrumentation innovations intended at meeting practitioners' demands has expanded in the late 1990s.Looking back on the creation and use of CE is intriguing. When electrophoresis was the standard technique of analysis and usage of CE was seen as an extension of earlier methods, the early uses of CE concentrated on the separation of biomolecules such as proteins and peptides.
Mass spectrometry is an effective method for discovering unknowns, researching molecular structure, and exploring the basic tenets of chemistry. Through the creation of gas-phase ions that are detected and identified by their mass and charge, mass spectrometry studies matter. [1,2]
II. CAPILLARY ELECTROPHORESIS AND MASS SPECTROMETRY HYPHENATION
This effective method for targeted or thorough high throughput studies of low-concentration analytes often occurring in complex sources, typical for bioanalytical applications, combines CE which is used for separation having high resolution with the MS which is having high-sensitivity and selectivity detections. [3]
According to their charge-to-size ratio, the components of a sample are separated using capillary electrophoresis in CE-MS. An electrolyte buffer-filled capillary is injected with the sample, and an electric field is then delivered across the capillary. Depending on their charge and size, the charged analytes move through the capillary at various speeds, causing their separation.[4]
The analytes are fed into the mass spectrometer for detection and identification after CE separation. The separated analytes are then fed into the mass spectrometer after being ionized, often by electrospray ionization (ESI) or air pressure chemical ionization (APCI). The ions are accelerated, divided into groups depending on their mass-to-charge ratios, and detected in the mass spectrometer. The analytes' identification and amount are revealed in the ensuing mass spectrum.[5]
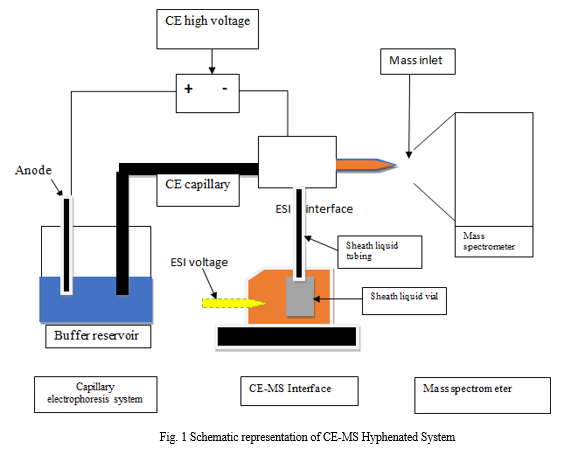
A. Instrumentation
The hyphenated approach known as CE-MS involves connecting the CE to the MS using long capillaries, which lengthens the analysis time. Additionally, there is a dearth of adequate volatile buffer that can work with the mass spectrometer which plays important role in CE-MS hyphenation.
- CE
a. Injector
b. Capillary
c. BGE vessel (background electrolyte)
d. High voltage supply
e. Electrode
f. Detector
2. MS
a. MS Interface
b. Analyzer
c. Detector
B. Coupling of Capillary Electrophoresis with Mass Spectrometer
The combination of capillary electrophoresis with mass spectrometry, or CE-MS, has emerged as an effective standard technique for the analysis of a wide variety of analytes. The most crucial parameter for selectivity in CE for coupling purposes is ph. The ability to directly couple these techniques to ESI-MS is made possible by the availability of volatile background electrolyte systems in CE that span practically the whole pH range.
For low pH values, formic acid and acetic acid are the most prevalent background electrolytes, while their respective ammonium salts are used for acidic to neutral pH values. Ammonium hydroxide (NH4OH) and ammonium carbonate ((NH4)2CO3) are additional volatile buffers for higher pH ranges. [6]
- Interfaces
The running buffer used in capillary electrophoresis, which has high ionic strengths and components with low volatility, is largely responsible for the quality of the analysis. While posing the fewest physical and chemical issues, an optimized interface should deliver good electrical contact. [7]
There are currently three types of interfaces which has been used:
a. Liquid junction
b. Sheathless
c. Co-axial liquid sheath
2. Sheath
An additional sheath liquid is used in sheath-flow interface, typically coaxially delivered by a capillary surrounding the metal needle, provides both a constant (electrolyte-independent) flow and an electrical contact. The sheath liquid, which serves as a solvent to stabilize and improve ESI signals, is hydrodynamically or externally injected into the ESI needle in its entirety. Sheath solutions are used to improve the ionization process as well as the electrical contact, and occasionally to help the solubility of the analytes being studied. The researched analyte has a significant impact on the sheath liquid's composition, which typically follows a few general guidelines: Due to a reduced surface tension, it is frequently necessary to create an aqueous solution with 50–80% of a moderately polar organic solvent (MeOH or ACN, for example) in order to produce a stable spray. [8]
III. PROTEIN SEPARATION BY CE-MS
A popular method for proteome analysis is CE-MS, a proteomic technology that combines chromatographic methods with MS to improve the resolving power. In a single step, the CE isolates the proteins with high precision based on their migration across a buffer filled capillary column in an electrical field of 300–500 V/cm.
Capillary zone electrophoresis (CZE has been employed generally in combination with MALDI-TOF or ESI-MS for the study of proteins and peptides. [9,10,11]
A. Materials and Methods
- Selection of Capillary
The separation performance of our CE-MS equipment with a variety of capillaries, including a non-coated capillary, a cationic polymer dynamic coating (polybrene coated capillary), a neutral permanent coating (Polyethylene glycol coated capillary), and a neutral permanent coating (commercial PVA coated capillary), in order to select a capillary which is having the ability to prevent the adsorption of proteins and peptides to the surface of the capillary. [12]
2. Buffers and Electrolyte
Type of capillary being used and its Mass compatibility determine the background electrolyte (BGE), also known as the running buffer, that should be utilized. For CE-MS investigations, volatile acid-base type buffers must be employed. CH?COOH, NH4HCO2, CH?O?, and C?H?NO? are a few of these. It is best to refrain from using nonvolatile buffers like H3PO4, H3BO3or surfactants as they may lead to the formation of impurities in the chamber which is using electrospray method, obstruct MS ionization, and subsequently reduce sensitivity of mass spectrometer.
For therapeutic proteins identification, CH?COOH and CH?O? are commonly used as background electrolyte, which makes them ESI-friendly because of their volatile nature. Additionally, acidic nature of BGE intact proteins and their digested peptides can be ionized and are hence accessible to positive ESI. [13]
B. Strength of Electric Field
The order of hundreds of volts (V) per centimeter high electric field, of the capillary, is required for CE to operate. If heat is not properly dissipated during high voltage, Joule heating can occur, which could result in a illustrative temperature profile over the capillary cross-section. [13]
Table 1 Components of CE-MS
|
Components |
Description |
Example |
|
Capillary electrophoresis |
|
|
|
Capillary |
Inner diameter: 50-75 µm Length: 30-60 cm For CE-MS (Length): 1m |
Fused silica capillaries |
|
Injector |
To inject the sample from the inlet end of capillary. |
|
|
Voltage |
A stable power supply of about 30kV (200-300mA) as a DC current is required for stable loading. |
|
|
Background electrolyte |
Based on electrolyte behavior a suitable buffer used for electro-osmotic and electrophoretic mobility which is having effective importance in ce-ms. |
|
|
Detectors |
Different detection devices used for detection of sample analyte separated by CE |
|
|
Mass spectrometer |
|
|
|
Interface |
For better separation and detection, a proper interface system is used.
|
|
|
Mass analyzer |
It is part of the mass spectrometer which isolate the ionized sample on the basis of their masses. |
|
|
Detector |
Creates a signal from incident ions by inducing a current caused by a moving charge or by producing secondary electrons that are then amplified. |
|

Table 2 Comparison of CE-MS with other hyphenated systems
|
Parameters |
LC-MS |
CE-MS |
|
Separation principle |
Based on adsorption between the analyte and stationary phase |
Based on electrophoretic mobility of analyte |
|
Separation |
Stationary phase |
Narrow capillaries |
|
Injecting sample quantity |
Micrograms |
Nanograms |
|
Flow rate |
Microliters per min. |
Nanoliters per min. |
|
Run time |
Several minutes to hours |
A few minutes |
IV. APPLICATIONS OF CAPILLARY ELECTROPHORESIS-MASS SPECTROMETRY
A. Protein Analysis and Proteomics
Capillary electrophoresis has the capacity to separate intricate combinations of analytes with minimal sample consumption. When LC and CE are both combine to MS via a sheath flow ESI interface, the second offers superior mass sensitivity and accelerates separations, while the former is less appropriate for analysis of diluted samples. A powerful tool for the examination of complicated biological samples is provided by connecting the highly effective CE with a system of detection that produces extensive data, like MS, via the gentle ESI mode. The need for such hyphenation is being highlighted by the growing interest in proteomics. In general, MS data are very desirable for identifying and tracking diagnostic marker peptides and proteins, as well as stability and purity studies.[15]
B. Metabolomics and Metabolite Analysis
Recent advancements have made capillary electrophoresis-mass spectrometry (CE-MS) a potent instrument for the study of charged species. The exceptionally high resolution and the ability to inject nearly any charged species into MS are the two main benefits of CE-MS. [16] Unlike other chromatographic techniques (GC or LC), which make use of the difference in interaction between the chemical substance to be used for analysis and the stationary phase. CE carry out separation based on differences in the mass-to-charge ratios (m/z), and neutral compounds are also isolate from ions. By observing ions over a range of m/z values, metabolites are selectively detected using MS after being first separated by CE. Numerous metabolites, particularly those involved in the central carbon metabolism, include charged carboxyl, amino, phosphate and hydroxyl groups, which makes them appropriate for CE-MS study. [17]
C. Monoclonal Antibodies
Due to the exceptional selectivity of the electrophoretic separation, capillary electrophoresis has thus proven to be a suitable analytical separation for the characterization and stability investigation of these types of protein. It is usual practice to evaluate mAbs using various electrophoretic modes, inclusive of capillary gel electrophoresis (CGE), capillary zone electrophoresis (CZE), and capillary isoelectric focusing. The majority of CE tests make use of optical detection. To get over the limits of optical detections, capillary electrophoresis hyphenated to mass spectrometry (CE-MS) has been widely explored to offer structural information.[18]
D. Chiral Analysis
CE has demonstrated significant potential for chiral compound enantioselective separation. CE is highly effective, requires little sample volume (a few nanoliters), and has quick migration periods. The on-line connection of CE to MS through ESI interfaces has been one of the key developments for the detection of chiral analytes in actual matrices. While MS/MS spectra can also provide data about the structure of the substance to be used for analysis, MS allows for the unambiguous assignment of the various electrophoretic peak types. Thus, chiral-capillary electromigration techniques and MS work together to create a very potent hyphenated technique that can offer excellent sensitivity and selectivity while also enabling the detection of unknown chiral chemicals in actual samples. [19]
V. ACKNOWLEDGEMENT
The authors would like to extend their sincere gratitude to all of the scientists whose work was cited in this article for their contributions to raising public understanding.
Conclusion
Capillary electrophoresis (CE) is an electrokinetic separation technique which allows the chemical separation of charged ions in aqueous or organic phase on the basis of differences in their velocity when an electric field is applied while the use of mass spectrometry (MS) to identify and characterize biological molecules in protein biochemistry and proteomic analysis. CE-MS is a technology that makes its compatible and intersecting place among the most classical chromatographic separation techniques coupled to MS (LC-MS, GC-MS) with a constant increase in recent years. CE-MS is having an ability to separate analytes present in extremely low concentration with high efficiency at high speed has made it applicable in all fields of science. Capillary Zone Electrophoresis coupled with Mass spectrometry nowadays is being a powerful tool for global multiomics and multilevel proteomics of cells because CZE can achieve highly efficient separations of metabolites, nucleotides, glycans, peptides, and protein complexes.
References
[1] K. D. Altria, “Overview of capillary electrophoresis and capillary electrochromatography,” vol. 856, pp. 443–463, 1999. [2] Altria, Kevin D. \"Overview of capillary electrophoresis and capillary electrochromatography.\" Journal of Chromatography A 856.1-2 (1999): 443-463. [3] Kleparnik, K., Electrophoresis 2015, 36, 159–178. [4] Haselberg, R., de Jong, G. J., & Somsen, G. W. (2013). CE?MS for the analysis of intact proteins 2010–2012. Electrophoresis, 34(1), 99-112. [5] Gaspar, A., Englmann, M., Fekete, A., Harir, M., & Schmitt?Kopplin, P. (2008). Trends in CE?MS 2005–2006. Electrophoresis, 29(1), 66-79. [6] Two-dimensional capillary electrophoresis-mass spectrometry (CE-CE-MS): coupling MS-interfering capillary electromigration methods with mass spectrometry [7] Ding, J., & Vouros, P. (1999). Peer Reviewed: Advances in CE/MS. Analytical Chemistry, 71(11), 378A-385A. [8] Pant??ková, P., Gebauer, P., Bo?ek, P., & K?ivánková, L. (2009). Electrolyte systems for on?line CE–MS: Detection requirements and separation possibilities. Electrophoresis, 30(1), 203-214. [9] D. Theodorescu, D. Fliser, S. Wittke, H. Mischak, R. Krebs, M. Walden, M. Ross,E. Eltze, O. Bettendorf, C. Wulfing, A. Semjonow, Pilot study of capillary electrophoresis coupled to mass spectrometry as a tool to define potential prostate cancer biomarkers in urine, Electrophoresis 26 (14) (2005) 2797–2808. [10] Figeys, D., & Aebersold, R. (1998). High sensitivity analysis of proteins and peptides by capillary electrophoresis?tandem mass spectrometry: recent developments in technology and applications. Electrophoresis, 19(6), 885-892. [11] Mikšík, I. (2019). Coupling of CE?MS for protein and peptide analysis. Journal of separation science, 42(1), 385-397. [12] Nguyen, T. T., Petersen, N. J., & Rand, K. D. (2016). A simple sheathless CE-MS interface with a sub-micrometer electrical contact fracture for sensitive analysis of peptide and protein samples. Analytica Chimica Acta, 936, 157-167. [13] Kumar, R., Rathore, A., & Krull, I. (2019). Practical Considerations for Capillary Electrophoresis–Mass Spectrometry for Analysis of Biotherapeutics. LCGC North America, 37(6), 386-391. [14] Martelli, C., & Desiderio, C. (2018). Capillary Electrophoresis-Mass Spectrometry for Proteomics. In Capillary Electromigration Separation Methods (pp. 335-351). Elsevier. [15] Ramautar, R., Heemskerk, A. A., Hensbergen, P. J., Deelder, A. M., Busnel, J. M., & Mayboroda, O. A. (2012). CE–MS for proteomics: Advances in interface development and application. Journal of proteomics, 75(13), 3814-3828. [16] Soga, T., Ohashi, Y., Ueno, Y., Naraoka, H., Tomita, M., & Nishioka, T. (2003). Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. Journal of proteome research, 2(5), 488-494. [17] Hirayama, A., Wakayama, M., & Soga, T. (2014). Metabolome analysis based on capillary electrophoresis-mass spectrometry. TrAC Trends in Analytical Chemistry, 61, 215-222. [18] Lechner, A., Giorgetti, J., Gahoual, R., Beck, A., Leize-Wagner, E., & François, Y. N. (2019). Insights from capillary electrophoresis approaches for characterization of monoclonal antibodies and antibody drug conjugates in the period 2016–2018. Journal of Chromatography B, 1122, 1-17. [19] Shamsi, S. A. (2002). Chiral capillary electrophoresis?mass spectrometry: Modes and applications. Electrophoresis, 23(22?23), 4036-4051.
Copyright
Copyright © 2023 Attish Bhardwaj, Aman Sharma, Upasana Thakur, Amar Deep Ankalgi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET54567
Publish Date : 2023-07-01
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

