Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Antimicrobial Activity of Biosynthesized Copper Nanoparticles Using Methanolic Extract of Ocimum Sanctum
Authors: Rishav Biswas, Pritha Sen, Dr. Priyanka Banerjee
DOI Link: https://doi.org/10.22214/ijraset.2024.63600
Certificate: View Certificate
Abstract
Methanolic extract of Ocimum sanctum leaves were used as a reducing and stabilizing agent for the synthesis of copper nanoparticles (CuNPs). It is a cost-effective and eco-friendly process. On the treatment of Ocimum sanctum leaf extract with copper sulphate solution, stable CuNPs were formed. The formed CuNPs were characterized under UV-Vis spectrophotometer. The biologically synthesized copper nanoparticles show high antibacterial activity against opportunistic pathogen Staphylococcus aureus. The antimicrobial activity was determined by three assays with agar well diffusion, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) and the values were compared to observe the antimicrobial efficacy of the CuNPs.
Introduction
I. INTRODUCTION
Nanoparticles are the materials that range in at least one of its dimensions from 1nm to 100nm. They possess unique properties from their bulk materials based on specific size, shape, composition and distribution. But the properties change according to the reduction in the dimensions of the materials to the molecular or atomic level [16]. The physical properties of a bulk material usually remain constant despite of its size but at the nanoscale level, bulk materials those are well characterized shows intriguing properties [20]. Copper is widely used and easily available material all over the world due to its low cost and high thermal conductivity as compared to other metals such as silver, gold, titanium, etc. The shapes of Copper Nanoparticles (CuNPs) are present in multiple forms such as spherical, rod shaped, triangular or tetrahedral shaped and in the form of flakes [5],[9],[14],[18]. Copper nanoparticles can easily undergo oxidation when it comes in contact with air or aqueous media to form copper oxides, so to maintain its stability it should be enclosed by organic or inorganic coating such as silica or carbon to protect it from oxidation [5],[6]. It has been reported that Copper nanoparticles that are obtained from plant extracts doesn’t require any coatings for stability because the plant extracts act as both reducing and stabilizing or capping agents during the synthesis of nanoparticles [8],[10],[11],[17]. Copper nanoparticles can be synthesized by bottom-up approach which includes both biological and chemical method. The source mainly used for synthesis of copper nanoparticles is from different plant extracts by reduction of metal ions to metallic form [3]. The biosynthesis of metallic nanoparticles is a bottom-up approach in which reduction/ oxidation of metallic ions occurs via biomolecules such as enzymes, sugars and proteins. The synthesis of nanoparticles by biological method is cost effective and eco-friendly compared to physical and chemical methods that often use toxic chemicals. On the other hand, Ocimum sanctum is a very reliable source for the biosynthesis of copper nanoparticles as Ocimum itself contains many different phytochemicals which help to treat vast range of diseases. The leaf volatile oil of Ocimum sanctum contains phytochemicals urosolic acid, eugenol, carvacrol, linalool, methyl carvicol, limatrol, euginal, caryophyllene and the seed volatile oil of the plant consists of sitosterol and fatty acids [7],[12]. Ocimum sanctum also prevents many diseases like allergy, inflammation, bronchitis etc. and acts as an important antimicrobial agent for treating antibacterial and antiviral diseases [12].
II. METHODOLOGY
For this study, Ocimum sanctum (Tulsi) leaves were taken and thoroughly washed with distilled water, dried in hot air oven and ground.
A. Preparation of Crude compound
50gm of dried ground tulsi leaves was dissolved in methanol. The solution was steamed for the phytochemicals to absorb by the solvent. It was then filtered was further used for the synthesis of copper nanoparticles.
B. Synthesis and Characterization of Copper Nanoparticles
For synthesis, equal volumes of copper sulphate solution and plant extract were incubated at room temperature in a dark chamber. Following incubation, the solution showed initial color change and pH was recorded. The wavelength (λ) was recorded by UV-vis spectrophotometer at a range from 600nm to 240nm and the presence of nanoparticle was confirmed.
III. ANTIMICROBIAL ACTIVITY OF COPPER NANOPARTICLES
Pure cultures of Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans were obtained from the Metropolis Lab of Ramaiah Memorial Hospital, Bengaluru. The techniques that were employed to study the antimicrobial activity were defined in 3 detailed assays.
A. Agar-well Diffusion Assay
Mueller Hinton agar (MHA) (Himedia, India) plates were used for this assay. YEPD media was used for culturing fungus. The petri-plates containing media were seeded using cotton swab with 24hr (old) culture of the microbial strains.
Wells of 8mm diameter were cut using sterile cork borer and 50 µL of different concentrations of test sample (CuNPs) were loaded in the wells of each plate to determine the antimicrobial potential. Ciprofloxacin and Clotrimazole were used as positive control at a concentration of 1mg/ml.
The plates were then incubated at 37°C for 24 hours. The antimicrobial activity was assayed by measuring the diameter of the zone of inhibition formed around the well.
B. Minimum Inhibitory Concentration (MIC) by Micro-dilution Assay
96-well microtiter plate (Thermo Fisher Scientific Inc., USA) was used for this set of experiments. To each well 100 µL of Mueller Hinton broth (MHB) was loaded and serial dilution was performed in which Ciprofloxacin was used as standard and added to the wells at a concentration of 1mg/ml.
Test sample (CuNPs) was added to the wells at a concentration of 20mg/ml. The experiment was done in triplicates. Staphylococcus aureus bacterial innocula was grown in MH broth and the suspensions density were adjusted to standard turbidity (7.5 x 105 CFU/mL). Microbial density was measured in the same manner as the antibacterial assay using a spectrophotometer. When the innocula reached the standard density, 5μL of the microbial suspension was added to each well from lowest to highest concentration of both CuNPs and Ciprofloxacin columns.
The microtiter plate was then incubated at 37oC for 24 hours. Following this, 100 µL of p-iodonitrotetrazolium (INT) violet (Loba Chemie, India) (0.4 mg/mL) was added to each well in the microtiter plate and was incubated for 30 minutes at 37oC. Wells showing red color indicated the bacterial growth (presence of turbidity) and wells with lowest concentration of test sample (Cu NPs) without any red color was determined as the minimum inhibitory concentration (MIC). Absorbance readings were taken using a microplate reader (Biobase, China) at 600nm.
C. Minimum Bactericidal Concentration (MBC) by micro-dilution Assay
This assay was performed in a similar manner to that of the MIC by micro-dilution assay. Instead of adding p-iodonitrotetrazolium (INT) violet, the culture was streaked on Luria Bertani (LB) agar plates from microtiter plate and was kept for overnight incubation at 37oC. Visual observation was done to determine the bactericidal concentration on LB agar plates.
IV. RESULTS
A. Synthesis and characterization of Copper Nanoparticles
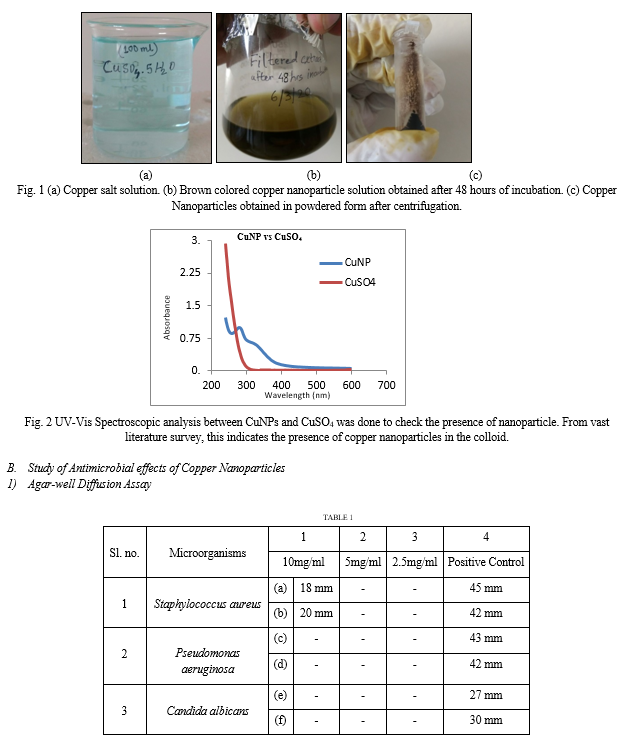
The first indication of synthesis of nanoparticle is the change in color of copper salt solution (CuSO4.5H2O) that is light blue in color Figure 1(a) whereas upon synthesis of nanoparticle the color changes to brown Figure 1(b) and it has been characterized by UV-Vis Spectrophotometer.
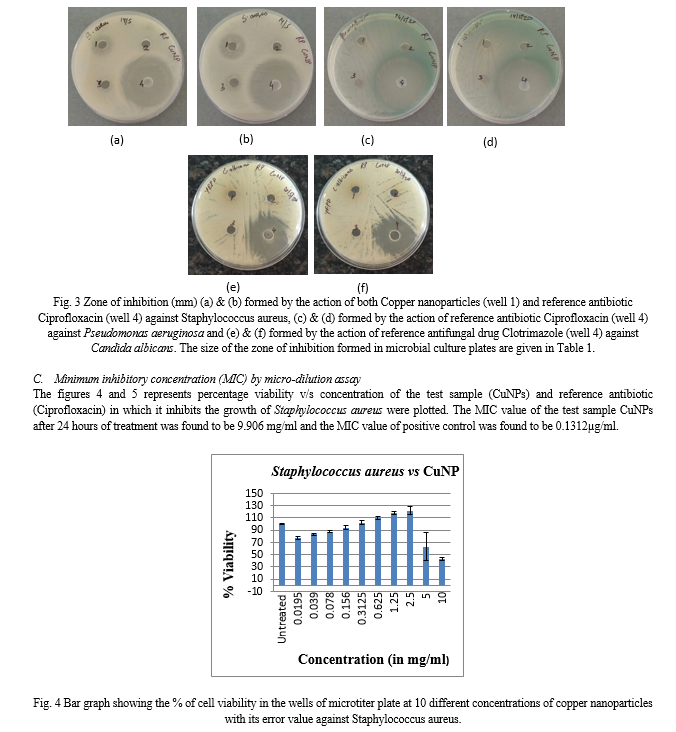
Shift in the peaks of pure copper salt solution and plant extract copper nanoparticle indicates the synthesis of nanoparticle. As shown in the Figure 2, the peak of copper nanoparticles was observed between 270 nm to 360 nm with absorbance of 0.9347 to 0.3282.
V. DISCUSSION
From the above results it can be clearly stated that the Ocimum sanctum leaf extract acts as reducing agent which reduces the copper sulphate solution to copper atoms in a bottom-up approach structurally to form copper nanoparticles. pH shift was observed from neutral pH before the synthesis to acidic pH (5.0) post synthesis of nanoparticles. The colloidal solution obtained after the filtration of 48 hours old mixed culture which is dark green in colour appears brown is due to the surface plasmon resonance effect which gives peaks at 286 nm and the copper sulphate solution at 240 nm upon scanning UV-Vis Spectroscopy indicates successful synthesis of copper nanoparticles as depicted in the Figure 2. This is in line with the work by Abboud et al. (2014) [1] who worked with the synthesis of Copper Nanoparticles. Surface plasmon resonance is utilized to detect molecular interactions such as interactions that occur between proteins or other classes of molecules. It measures the binding of molecules in real-time without the use of labels. As the literature survey says that the phytochemicals and phenolic compounds of Ocimum sanctum leaf extract play an important role as a reducing agent for the synthesis of copper nanoparticles [2],[13],[15],[18],[21].
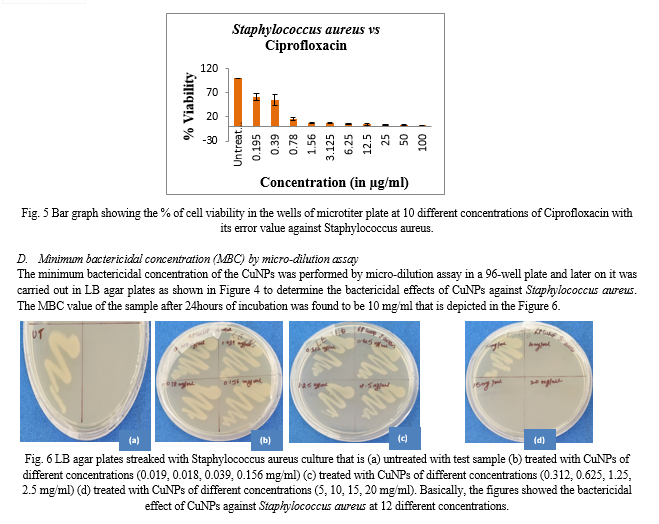
In the present study, the antimicrobial activity of CuNPs i.e. derived from Ocimum sanctum leaf extract was examined against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. First, agar-well diffusion method was performed in duplicates in which CuNPs showed effective zone of inhibition against S. aureus (18mm and 20mm) only which was compared with a reference antibiotic Ciprofloxacin (positive control) as represented in the Figure 3.
The CuNPs showed susceptibility only against Gram positive bacteria (S. aureus) as compared to Gram negative bacteria (P. aeruginosa) and a fungal strain (C. albicans) as shown in Table 1. Several independent studies have reported that diffusion method is not the proper method to determine the exact concentration of a drug [4]. To confirm these, micro-dilution assays were performed.
Micro-dilution assays were performed only on S. aureus to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the CuNPs (test sample) because in diffusion assay it did not show any result against the other two pathogenic microbes. The result obtained in MIC (9.906 mg/ml) was almost close to the concentration of well diffusion assay which shows the bacteriostatic effect of CuNPs. The result obtained in MBC (10 mg/ml) is same as the concentration level of well diffusion assay which shows the bactericidal effect of CuNPs. In brief, the results obtained in all the three assays showed that bacteriostatic and bactericidal effects are almost in same concentrations. Though the mechanisms of action of metal nanoparticles are not fully understood yet, but it can overcome the rising drug resistance problem against the microorganisms.
Conclusion
Researchers are constantly exploring for a way to find better antimicrobial agent that can be used as medicine as well as in other commercial products for e.g. in toothpaste, hand-wash, ointment, etc. The metal nanoparticles being excellent antimicrobial agent fulfill that purpose. With the increase of novel emerging diseases, it is necessary for scientists to come up with a novel antibiotic or antifungal drug which will work efficiently against those challenging microbial diseases. This paper focuses on the biosynthesis of copper nanoparticles from Ocimum sanctum leaf extract. Among the other synthesis methods, green synthesis has proved to be much more efficient, eco-friendly and cost-effective [11],[13]. The synthesized copper nanoparticle was characterized by UV-Vis spectrophotometer and used as antimicrobial agent against the microbes Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Agar-well diffusion method and MIC followed by MBC showed promising results against Staphylococcus aureus. The intention of this work is to explore the antimicrobial efficacy of copper nanoparticles against opportunistic pathogens and establish the green synthesis method from a clinically beneficial plant source. Worldwide research and development of metal nanoparticles is being accelerated to meet the demands of medicines for new emerging microbial diseases. The researchers are yet to see many future applications of metallic nanoparticles.
References
[1] Abboud Y., Saffaj T., Chagraoui A., El Bouari A., Brouzi K., Tanane O., Ihssane B. “Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata)”. Appl Nanosci (2014) 4:571–576. DOI 10.1007/s13204-013-0233-x. [2] Agarwal V. “Anti-fungal properties of Ocimum sanctum Linn: A short review”, Journal of Medicinal Plants Studies 2015; 3(5): 74-75. [3] Annapurna S., Suresh Y., Sreedhar B., Bhikshamaiah G., Singh AK. “Characterization of Green Synthesized Copper Nanoparticles Stabilized by Ocimum Leaf Extract”, Mater. Res. Soc. Symp. Proc. Vol. 1704 © 2014 Materials Research Society DOI: 10.1557/opl.2014.806. [4] Balouiri M., Sadiki M., Ibnsouda SK. “Methods for in vitro evaluating antimicrobial activity: A review”. Journal of Pharmaceutical Analysis 6 (2016) 71–79. [5] Caroling G., Priyadharshini MN., Vinodhini E., Ranjitham AM., Shanthi P. “Biosynthesis of Copper Nanoparticles using aqueous guava extract– Characterisation and Study of antibacterial effects”, IJPBS Volume 5, Issue 2, APR-JUN 2015, 25-43. [6] Chatterjee AK., Chakraborty R., Basu T. “Mechanism of antibacterial activity of copper nanoparticles”, Nanotechnology 25 (2014) 135101 (12pp), doi:10.1088/0957-4484/25/13/135101. [7] Devi P U. “Radioprotective, anticarcinogenic and antioxidant properties of the Indian Holy Basil, Ocimum sanctum (Tulasi)”, Indian Journal of Experimental Biology, Vol.39, March 2001, pp.185-190. [8] Din MI., Arshad F., Hussain Z., Mukhtar M. “Green Adeptness in the Synthesis and Stabilization of Copper Nanoparticles: Catalytic, Antibacterial, Cytotoxicity, and Antioxidant Activities”, Nanoscale Research Letters (2017)12:638, DOI 10.1186/s11671-017-2399-8. [9] Gultekin DD., Gungor AA, Onem H, Babagil A, Nadaroglu H. “Synthesis of Copper Nanoparticles Using a Different Method: Determination of Their Antioxidant and Antimicrobial Activity”, JOTCSA. 2016; 3(3):623–36. [10] Hussain I N., Singh B., Singh A., Singh H., Singh S C. “Green synthesis of nanoparticles and its potential application”, Biotechnol Lett (2016) 38:545–560. [11] Iravani S. “Green Synthesis of Metal Nanoparticles using plants”. Green Chem., 2011, 13, 2638–2650. [12] Kelm M.A., Nair M.G., Strasburg G.M., DeWitt D.L. “Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn”, Phytomedicine, Vol.7(1), pp. 7-13, 2000. [13] Kulkarni VD., Kulkarni PS. “Green Synthesis of Copper Nanoparticles Using Ocimum Sanctum Leaf Extract”, International Journal of Chemical studies, 2013. [14] Lee H J., Song J Y., Kim BS. “Biological synthesis of copper nanoparticles using Magnolia kobus leaf extract and their antibacterial activity”, J Chem Technol Biotechnol (2013) DOI 10.1002/jctb.40 52. [15] Prasannabalaji N., Muralitharan G., Sivanandan RN., Kumaran S., Pugazhvendan SR. “Antibacterial activities of some Indian traditional plant extracts”, Asian Pacific Journal of Tropical Disease (2012) S291-S295. [16] Rai RV., Bai JA. “Nanoparticles and their potential application as antimicrobials”, FORMATEX, 2011. [17] Shameli K., Ahmad M B., Jaffar Al-Mulla E A., Ibrahim N A., Shabanzadeh P., Rustaiyan A., Abdollahi Y., Bagheri S., Abdolmohammadi S., Usman M S., Zidan M. “Green Biosynthesis of Silver Nanoparticles Using Callicarpa maingayi Stem Bark Extraction”, Molecules 2012, 17, 8506-8517; doi:10.3390/molecules 17 078506. [18] Shankar S., Rhim JW. “Effect of copper salts and reducing agents on characteristics and antimicrobial activity of copper nanoparticles”, Materials Letters, doi:10.1016/j.matlet.2014.06. 014. [19] Tamilvanan A., Balamurugan K., Ponappa K., Kumar BM. “Copper Nanoparticles: Synthetic Strategies, Properties and Multifunctional Application”, International Journal of Nanoscience Vol. 13, No. 2 (2014) 1430001 (22pages) DOI: 10.1142/S021 95 81X14300016. [20] Thakkar KN., Mhatre SS., Parikh PR. “Biological synthesis of metallic nanoparticles”. Nanomedicine: NBM 201 0;6:257-262, doi:10.1016/j.nano.2009.07 .002. [21] Verma S., “Chemical constituents and pharmacological action of Ocimum sanctum (Indian holy basil-Tulsi)”, JPhytopharmacology2016;5(5):205-207.
Copyright
Copyright © 2024 Rishav Biswas, Pritha Sen, Dr. Priyanka Banerjee. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63600
Publish Date : 2024-07-10
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

