Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Microbial Production Bioemulsifier and its Applications
Authors: Mr. Gopani Juvin Shaileshbhai, Ms. Trupti Pandya
DOI Link: https://doi.org/10.22214/ijraset.2023.54727
Certificate: View Certificate
Abstract
According to various research, Biosurfactants are generally composed of amphipathic motes that have both hydrophilic and hydrophobic ingredients. The hydrophilic composites generally correspond to positive, negative, or amphoteric charged ions, whereas the hydrophobic composites are made up of a long chain of fatty acids. It is getting important in biotechnology products for numerous industrial applications including in food, cosmetics and cleaning products, medicine, drug, and oil and gas. Bacterial cells produce an admixture of biosurfactant( BS) lipids with the help of which oil is dispersed into veritably fine droplets and therefore the bioavailability of CO is increased. Biosurfactants are surface-active site compounds produced by microorganisms. Biosurfactant generally refers to surfactants of microbial origin. Most of the biosurfactants produced by microbes are synthesized extracellularly and numerous microbes are known to produce biosurfactants in large relative amounts. This study concentrates on the insulation of biosurfactant-producing microorganisms from soil samples.
Introduction
I. INTRODUCTION
Chemical substances known as surfactants are made up of amphipathic molecules with hydrophilic and hydrophobic moieties that separate at physical contacts. The polar moieties can be cationic, anionic, nonionic, or amphoteric molecules, whereas the non-polar moieties are frequently chains of hydrocarbons. Surfactants can create microemulsions, in which hydrocarbons are soluble in water or vice versa, and lower surface and interfacial tensions thanks to the mix of hydrophobic and hydrophilic moieties.[1] Because they can increase the aqueous solubility of Non-Aqueous Phase Liquids (NAPLS) by lowering their surface/interfacial tension at the air-water and water-oil interfaces, surfactants, the active ingredients in soaps and detergents, are frequently used to separate oily materials from a particular media.[2]. Surface-active chemicals produced by microorganisms called biosurfactants, also known as surface-active substances, either attach to cell surfaces or are discharged extracellularly in the growth media.[3] The primary classifications of biosurfactants are based on their chemical makeup and microbiological source. Glycolipids, phospholipids, polymeric biosurfactants, and lipopeptides (surfactin) are the four primary groups of biosurfactants. Rhamnolipids, also known as phorolipids and trehalolipids, are the most well-known glycolipids. Both aqueous solutions and hydrocarbon mixes have lower levels of surface tension and Critical Micelle Dilution (CMD) due to these molecules. These characteristics lead to the creation of micelle formations, which allow hydrocarbons to dissolve in water or in water-soluble hydrocarbons. [4] Surfactants are widely employed in industrial, agricultural, food, cosmetic, and medicinal applications. However, the majority of these molecules are chemically synthesised, and because of their refractory and persistent nature, they may pose environmental and toxicological risks.
Recent developments in biotechnology have drawn attention to an alternate, environmentally acceptable method for producing several kinds of biosurfactants from microorganisms. [5] Biosurfactant-producing microorganisms are abundant in nature and can be found in soil, sediment, and sludge as well as freshwater, groundwater, and the sea. Additionally, they can be found in harsh environments (oil reservoirs), and they can survive in a variety of temperature, pH, and salinity conditions. [6]
Additionally, they are capable of being removed from environments that are undisturbed and in which they perform physiological functions that do not involve the solubilization of hydrophobic contaminants, such as antibacterial activity, biofilm formation, or processes of motility and surface colonisation. [7]
A. Biosurfactant's Physiological Function in Microorganisms
A number of microorganisms produce biosurfactants, which are primarily released extracellularly or linked to cell components during growth on water-immiscible substrates [8]. Although the molecular mechanisms relating to the uptake of their substrates are still unclear and incompletely understood, the main physiological function of biosurfactants is to enable microorganisms to grow on water insoluble substrates by reducing the surface tension at the phase boundary. This makes the substrate more readily available for uptake and metabolism. [8,9]
The evidence that emulsification is a natural process induced by extra-cellular agents is indirect, and there are some conceptual challenges in comprehending how emulsification can provide a (evolutionary) advantage for the microorganism producing the emulsifiers. It has been suggested that when the surface area becomes limiting, biomass increases arithmetically rather than exponentially. The antibacterial properties of biosurfactants towards different microorganisms serve as another physiological function. Different surfactants typically hinder various taxa. In addition, biosurfactants have been demonstrated to play a role in virulence, cell desorption, and cell adhesion, which confers the most stability under adverse environmental circumstances [8].
Biosurfactants have been produced using substances such as urea peptone, yeast extract, ammonium sulphate, ammonium nitrate, sodium nitrate, meat extract, and malt extracts. Although the most widely used nitrogen source for the production of biosurfactants is yeast extract, the concentration of its use depends on the organism and the growth medium. Arthrobacter paraffineusfavours ammonium salts and urea as nitrogen sources for the generation of biosurfactants, whereas P. aeruginosa prefers nitrate for optimum surfactant synthesis [10].
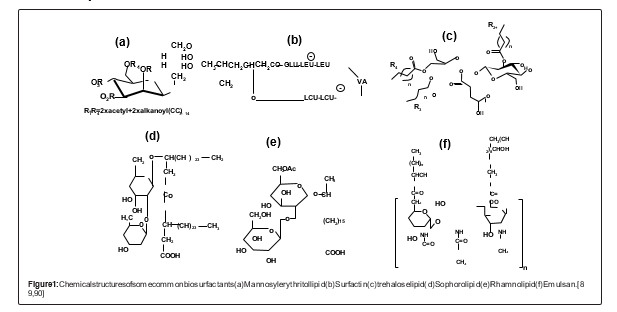
B. Producing Biosurfactants is Impacted by Certain Factors
Either by excretion or attachment to cells, biosurfactants are made. Through the lowering of surface tension between phases, which increases the availability of hydrophobic substrate for uptake and metabolism, biosurfactants' primary physiological function is to promote access to or enable microbial cells to develop on insoluble substrates. These substrates' various uptake processes are outlined. Direct contact between cells and large hydrocarbon droplets, direct ingestion of dissolved hydrocarbons in the aqueous phase, and interaction with emulsified droplets (emulsion) have all been documented. Additionally, biosurfactants play a role in how bacteria adhere to hydrocarbons. These carbon sources can support microbial growth due to cell adsorption to insoluble substrates and surfactant compound excretion [11].The best biosurfactant yield can be difficult to achieve since a variety of factors can affect the development and metabolism of microorganisms during fermentative synthesis. Numerous studies have been conducted to determine the best mix of substrates for a defined culture medium to promote intracellular diffusion and the synthesis of chemicals of interest.[11,12,13].
It's crucial to specify the growth conditions for a chosen strain of microbe in order to get the best possible biosurfactant production. Considerable factors include supplies of carbon and nitrogen, lipophilic substrate content, availability of micronutrients, inoculum size, temperature, pH, aeration, and agitation speed [14].Although the majority of biosurfactant-producing microorganisms produce these substances under more constrained circumstances, it is important to look into the growth phase (exponential or stationary phase) that results in the highest production rate. [11]In order to find the best culture conditions for the highest production of biosurfactants at the lowest possible cost, the chemical and physical parameters of the fermentation process can be optimised using statistical methods, which give the chance to study the effects of interactions between the different variables [15,16].
The method must incorporate upstream production and downstream processing in order to create biosurfactants economically. Mechanisms to increase production must be taken into account, including new statistical techniques (like surface methodology), AI-based methods like Artificial Neural Intelligence combined with Genetic Algorithm (ANN-GA), and the use of recombinant bacterial strains. Most recently, Ambaye et al. [17] Cost-effective large-scale industrial biosurfactants production for the importance of the environment can be achieved through the use of genetically modified microbial strains, cost-effective substrate(s), optimised media, improved fermentation processes, better downstream processing, and purification of end products.
C. Carbon Sources
The kind of carbon substrate has an impact on and influences the quantity and quality of biosurfactant production. [18] According to reports, a good source of carbon substrate for the generation of biosurfactants is diesel, crude oil, glucose, sucrose, and glycerol [19].
D. Sources of Nitrogen
Since protein and enzyme synthesis is dependent on nitrogen, nitrogen is vital in the biosurfactant production medium. The creation of biosurfactants has utilised a variety of nitrogen compounds, including urea peptone, yeast extract, ammonium sulphate, ammonium nitrate, sodium nitrate, meat extract, and malt extracts. Although yeast extract is the most popular nitrogen source for making biosurfactants, its use in terms of concentration depends on the organism and the culture medium. Arthrobacter paraffineus prefers ammonium salts and urea as nitrogen sources, whereas P. aeruginosa uses nitrate to support maximal surfactant synthesis [20].
E. Environmental Variables
These play a huge role in the yield and properties of the biosurfactant that is produced. As the product may be impacted by variations in temperature, pH, aeration, or agitation speed, it is always required to optimise the bioprocess in order to generate significant quantities of biosurfactants. According to reports, the majority of biosurfactant production takes place in a temperature range of 25–300C [21]. He said that 8.0, the pH of sea water in its natural state, was the optimum for producing. Aeration and Agitation: As both aid in the transport of oxygen from the gas phase to the aqueous phase, aeration and agitation are significant elements that affect the generation of biosurfactants. It has been hypothesised that the creation of bioemulsifiers can improve the solubilization of water-insoluble substrates and subsequently ease nutrient delivery to bacteria. This may also be related to the physiological function of microbial emulsifiers. Bednarski and Adamczak [20]It was noted that when the air flow rate was 1 v/m and the dissolved oxygen concentration was maintained at 50% of saturation, the best production value of the surfactant (45.5g/l) was attained. Salt concentration: Because salt concentration affects the cellular activities of microorganisms, it also has a comparable impact on the synthesis of biosurfactants in a particular medium. Contrary findings were seen for some biosurfactant compounds, however, which were unaffected by concentrations up to 10% (weight/volume), despite small CMC reductions being found [19].
F. Formulation of the Production Media for Biosurfactants
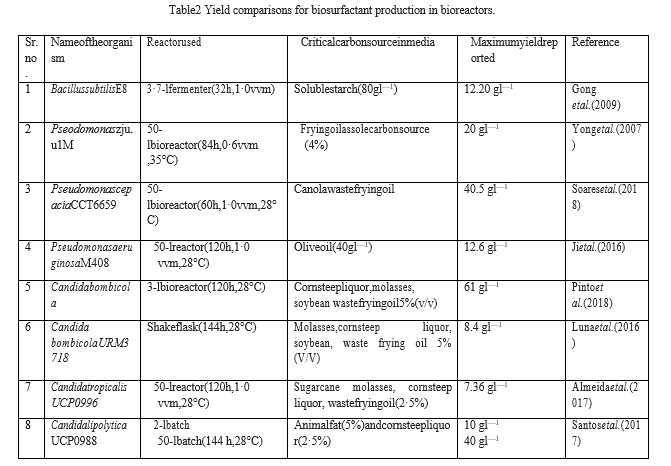
Since there are many connected factors involved in cell-based bioprocesses, monitoring each parameter independently becomes tiresome, time-consuming, and expensive. to manage numerous data sets simultaneously Media optimisation techniques such as Response Surface Methodology (RSM) and statistical techniques like Taguchi and Plackett-Burman Design have frequently been utilised to improve biosurfactant production procedures [22]. Table 1. In addition to statistical techniques, an AI-based technology called Artificial Neural Intelligence paired with Genetic Algorithm (ANN-GA) has also been tested for media optimization [23]
Table1 Different low-cost waste substrate sex pointed in recent times for biosurfactant production.
|
Sr.no. |
Typeofindustry/waste |
Typeofmicrobialspecies |
References
|
|
1 |
Food and Agroindustrial residue (datemolasses,sugarcanebaggasse,orangepeel, sesame peel flour, tuna fishresidue,bananapeel,potatopeel,cornsteep liquor, peanut oil cake, cassavawaste,moringa residue) |
Halobacteriaceae archaeon, Bacilluspumilis, Bacillus licheniformis,Cunninghamellaphaeospora,Candidatropicalis, Pseudomonasaeruginosa |
Chooklinetal.(2014);Sharma etal.(2015);Kumaretal.(2016);Linsetal.(2016); Rubio-Ribeauxet al. (2017);Magalh~aesetal.(2018) |
|
2 |
AnimalWaste(slaughterhousewaste,animalfats,fishprocessingwaste) |
Pseudomonas gessardii, Nocardiahigoensis, Aneurinibacillusmigulanus |
Ramanietal.(2012);Patiletal.(2016);Sellamietal.(2016) |
|
3 |
Agroindustrialandmillwaste(includingrefinery waste) (olive mill waste,tannery pretreated effluent, palm oilindustry waste, soybean oil industrywaste) |
Brachybacterium,paraconglomeratum,Pseudomonas aeruginosa, Bacilluspseudomycoides, Pseudomonasaeruginosa,Bacillussubtilis |
Kiranetal.(2014a);Gudin~aetal. (2016); Li et al. (2016); Moya-Ram´?rezetal.(2016);Radzuanetal.(2017) |
|
4 |
Wastecookingoil(wastefryingcoconutoil, wastecookingoil) |
Pseudomonasaeruginosa,Candidalipolytica |
GeorgeandJayachandran(2013);Lanetal.(2015);Souzaetal.(2016) |
In the past ten years, a lot of research has been done on media optimisation, particularly for the most well-known biosurfactant producers, the Pseudomonas, Bacillus, and Candida species. The kind and quantity of the media's carbon and nitrogen sources, as well as the type and ratio of the metal cations, have all been thoroughly investigated and proven to be crucial for the formation of biosurfactants in both shaking flasks and large-scale fermenters. Table 2 provides a summary of some of the significant carbon sources that, when cultures were cultivated in shaking flask and large-scale fermenter containers, generated the greatest reported biosurfactant output. The most affordable and lucrative substrates for industrial-scale biosurfactant production, namely for Pseudomonas, Bacillus, and Candida species, are vegetable oil and hydrocarbon-based substrates [24].

Rhamnolipid synthesis was encouraged by low magnesium concentration and optimal iron (Fe) concentration. In one of the most recent studies, blackstrap molasses was employed as the carbon source and RSM was used to optimise medium for the generation of rhamnolipids using the Pseudomonas aeruginosa strain (Raza et al. 2016). Total sugar (TS), carbon: nitrogen (C: N) ratio, and incubation time were the variables under observation. RSM provided a representation of the link between the intended response and independent input factors.All of the parameters were found to have an impact on the rhamnolipid yield, which was determined to be at its highest at TS 2%, C:N = 20, and T = 5 days. For increasing the production of rhamnolipids, the use of organosulfur compounds in the production medium has produced encouraging results. Surface tension was observed to be reduced more effectively in the presence of 4, 6-dibenzothiophene (DBT) by a sulfur-using strain of P. aeruginosa. Moreover, the rhamnolipid congeners generated varied depending on the sulphur compound used. Thus, it was discovered that sulphur had a significant impact on the quantity and variety of rhamnolipid congeners generated [27]. These strains also have the potential to be employed in a coproduction setup, which combines the production of biosurfactants with another bioprocess—in this example, desulphurization—to lower the total cost of the process. A rich mineral salts medium with nonlimiting carbon and nitrogen sources, a pH of almost zero, and the availability of crucial metal cations has been determined to be most beneficial for the formation of lipopeptides because lipopeptide production was discovered to be growth-associated as well. [28] It has been discovered that the bioavailability of iron (Fe) and manganese (Mn) salts in the media is essential for boosting the formation of biosurfactants of the lipoprotein type. Recent research on adding crude glycerol and Mn to agricultural feedstock led to improved growth and 793 mg/l of crude lipopeptide [29]. It was hypothesised that Mn would increase the microbial culture's use of ammonium nitrate, increasing the availability of free amino acids for surfactin production. According to several investigations, lipopeptides have chelating properties that reduce the amount of metal source that is available for development in the medium. Therefore, it has been discovered that occasional feeding of Fe and Mn promotes development. However, a number of elements interact together to influence both the quality and amount of biosurfactant production, and all of these combinations must be considered when creating the ideal media. In parallel, it becomes crucial to hunt for areas that have not been thoroughly investigated for improved biosurfactant production.
II. BIOSURFACTANTS APPLICATIONS
A. Oil Industries
The total daily oil consumption in 2018 increased by 1.5% over the previous year to 99.5 million barrels [30]. At the current pace of usage, light and medium oils should become more difficult to get, increasing reliance on heavy and extra-heavy oils. Additionally, it is anticipated that within the following 40 to 45 years, all remaining oil reserves would be exhausted [31]. In order to increase production, extend the time it takes to explore reserves, and gain access to oil residues trapped in rock pores, which are thought to make up about 60% of the oil in reservoirs, the oil industry is constantly looking for technological advancements. Primary, secondary, and tertiary procedures are often used to carry out the oil recovery process. [32]
Primary, secondary, and tertiary procedures are typically used to carry out the oil recovery process [33]. The primary and secondary approaches each include natural and induced pressure, while the tertiary method includes enhanced oil recovery (EOR) operations. In order to improve oil output and extend the life of decreasing reservoirs, EOR uses heat, the injection of miscible gas, and intriguingly, surfactants that are synthetically generated [34]. In order to recover secondary oil from sediments, microbial enhanced oil recovery (MEOR) includes substituting biologically derived secondary metabolites for synthetic surfactants. These biologically derived secondary metabolites include acids, biopolymers, enzymes, gases, solvents, and the most promising biosurfactants [35,36,37,38].
In order to promote microbial development during MEOR, nutrients and microorganisms that create biosurfactants are added to the oil reservoir [39,40]. By encouraging the drop in surface tension between the oil and rock, which lessens the capillary pressures that prevent the passage of oil through rock pores, biosurfactants are effective at mobilising immobile hydrocarbons [41,42]. In MEOR simulations using the biosurfactant made by B. amyloliquefaciens, Alvarez et al. [43] attained a petroleum hydrocarbon recovery rate of more than 90%. Using the foam fractionation approach and the biosurfactant generated by Bacillus sp. GY19, Khondee et al. [42] were able to extract 100% of the oil. Using the synthetic surfactants examined in the study [44] and biosurfactants derived from B. subtilis strains [45]. Reported higher recovery rates of 6-25% for heating oil, 16-24% for viscous paraffin oil, 13-18% for light Arabic oil, and 15-17% for heavy crude oil, demonstrating the effective recovery of residual oil from reservoirs exploited for lengthy periods of time. The recovery rate of medium weight oils increased by 11.91% when P. aeruginosa's rhamnolipid was present, leading to a 50.45% recovery rate, which was higher than the recovery rates attained with the synthetic surfactants used in the study [44]. The amount of biosurfactants needed to remove oil residues trapped in the porous rock may make the process unprofitable, making the use of biosurfactants in MEOR a contentious matter. Additionally, it is seen to be counterintuitive to use compounds for oil recovery whose primary value proposition is to replace synthetic chemicals from the petrochemical industry [46, 35].
The same qualities that are helpful for oil exploration can also be employed for bioremediation, which is frequently required due to accidents and the resulting hydrocarbon contamination of the environment [47,48].Biosurfactants can sustain a high rate of biodegradation in polluted soils, which makes them a good ecological alternative to synthetic surfactants, according to bioremediation methods. Biosurfactants can be released in situ, where they can carry out their effects with less subsequent handling work and are technically effective, as opposed to its synthetic equivalent [49]. With the added benefits of low toxicity and biocompatibility, biosurfactants promote the dispersion of contaminants in the aqueous phase and increase the bioavailability of the hydrophobic substrate to microorganisms for the subsequent removal of such pollutants through biodegradation [50].In addition, because of the repulsion between its major groups and soil particles caused by its adsorption on the contaminant surface, the release of pollutants from the soil is favoured. In the second scenario, pollutants that are incorporated into micelles are more likely to partition into the aqueous phase. The wash solution containing the surfactant can be recycled, which lowers cleanup costs, while pollutants partitioned into micelles can be recovered and demulsified, or even electrochemically destroyed or adsorbed on activated carbon [49].
According to Jadhav et al. [51], adding a biosurfactant made by Oceanobacillus sp. improved crude oil biodegradation by up to 90%. Using the biosurfactant created by B. brevis, Mouafi et al. [52] successfully achieved excellent dispersion and emulsification of motor oil in water. Numerous studies have shown the application potential for the cleanup of oil-contaminated soil. Surfactin is one of the many surfactants utilised in biotechnological decontamination procedures, with clearance rates of biomolecules produced by B. licheniformis exceeding 85% and those produced by B. subtilis above 88% [53,54,43]. There have been other biosurfactants utilised successfully in soil remediation by species of Bacillus, Pseudomonas, and Candida [55,56,57]. Hentati and others [58] It has recently been demonstrated that Pseudomonas aeruginosa'sglycolipidic biosurfactant is capable of removing hydrocarbons from contaminated soil.
The biosurfactant outperformed the studied chemical surfactants in terms of effectiveness. As with MEOR, there are concerns about whether microbial fermentation will be able to create enough biosurfactants to carry out bioremediation over the vast regions of hydrocarbon-contaminated land or ocean caused by either extended industrial usage or unintentional discharge. Although numerous studies have demonstrated that biosurfactants are an appealingoption for removing and/or improving the degradation of hydrophobic contaminants in soil, it is important to keep in mind that they, along with their synthetic counterparts, may have no effect or even delay degradation by inhibiting microbial metabolism [49, 55].
B. Detergent Industry
Products for personal care, household cleaning, and tough industrial cleaning are all part of the detergent market. The petrochemical industry typically provides the surfactant chemicals utilised in formulations in this business. For instance, during the COVID-19 epidemic, the most commonly used surfactant in the personal care and home care industries was derived from crude oil, which makes biodegradation challenging and poses a significant hazardous risk to aquatic habitats [59]. Biosurfactants are increasingly emerging as a viable commercial replacement for these synthetically generated surfactants [60].This predicament has sparked the hunt for environmentally friendly goods, such as straight-chain (non-branched) chemical compounds used in biodegradable detergents that facilitate effective microbial decomposition [61]. Replace synthetic surfactants with green surfactants, such as biosurfactants, and particularly those that are effective at low temperatures and/or in hard water, as this is a deliberate strategy to develop more sustainable detergents [62,63].The ability of biosurfactants to emulsify, which is important for detergent activity, is one of their key characteristics in this field. Other qualities, in addition to this one, are comparable to commercial detergents and can be used in the laundry and detergent sectors [64,68]. A lipopeptide from B. subtilis SPB1 was found to be more effective than conventional detergents at removing vegetable oil and coffee stains, according to Bouassida et al. [66]. Fei and co. [67]10,000 different cosmetic products' performance were examined, and 25–30% of these products were reformulated annually. The use of new active ingredients for the market or the industry is a factor in about 10% of these reformulations. Each year, these businesses add up to 80 new substances to their product line [68]. In this situation, using biosurfactants is one way to satisfy the demand for new components. Biosurfactants represent minimal dangers to humans and the environment due to their renewable, biodegradable, low-toxic, or non-toxic nature, which is in accordance with the interests of the developing consumer market and, as a result, the cosmetic business.
The development of safer, more inventive cosmetics has a good potential of being directly impacted by investments in the applied research of these biomolecules [69]. Cosmetics require the foaming, wetting, dispersing, and solubilizing qualities that biosurfactants naturally possess. Additionally, foaming is a desired quality for shampoos, soaps, and shaving creams. Water-in-oil creams with wetting capability can permeate the skin more readily. To combine pigments into a variety of goods, including nail paint and hair dyes, dispersing and solubilizing capacity is required [70,71]. Glycolipid and lipopeptide biosurfactants in general, which have antimicrobial, skin surface moisturising, and low toxicity properties that could make them suitable substitutes for chemical surfactants in current cosmetic and personal skincare pharmaceutical formulations, are examples of microbial biosurfactants that are applicable to the cosmetic industry [72]. Specific effects have also been reported such as moisturising properties (mannosylerythritol lipid), antiviral and antibacterial action (trehalolipids), increased dissolution of immiscible compounds in water (sophorolipids), moisturising and stabilising properties (emulsan), photoprotective potential (amino acids similar tomycosporin), foaming (surfactin), and mucosal re-epithelialization (rhamnolipids) [73,74].
C. Pharmaceutical and Medical Sector
Because of their antibacterial, anti-adhesive, and enzyme-inhibiting capabilities, biosurfactants have been utilised in the pharmaceutical and medical industries for various therapeutic applications [75]. Gene-releasing biosurfactants, medications, as well as antiviral and anticancer activity, are some of the key areas of research on these biomolecules in medicine and pharmacy [76]. By limiting the adhesion of dangerous bacteria, these features also offer new therapeutic methods to the prevention and treatment of illnesses and infections [74]. Giri [77]. found that biosurfactants, such as lipopeptides and glycolipids, can harm cell membranes, causing lysis and death as a result, preventing the growth of cancer cells. The antibacterial, anticancer, and anti-mycoplasma capabilities of surfactin are present. Because of their capacity to scavenge free radicals, lipopeptides have also been linked to improved tissue development and epidermal differentiation as well as decreased inflammation and wound healing [78]. Rhamnolipids or sophorolipids biosurfactants from Pseudomonas aeruginosa, Burkholderiathailandensis, and Starmerellabombicola were reported to contribute to oral hygiene by removing bacterial biofilms or inhibiting other bacterial cultures in the oral cavity [79, 80]. This is an additional application in oral health.
D. Agricultural
The adaptable qualities of biosurfactants also made it possible for them to be used in agriculture, primarily to replace synthetic surfactants in the formulations of pesticides and agrochemicals, favouring the expansion of "green chemistry" in this sector in response to the need to minimize/eliminate harmful effects on the environment and human health caused by the excessive use of chemical compounds [81,82].
According to the literature, rhamnolipid and lipopeptide biosurfactants work as bioremediation agents for soils, surface water, groundwater, and waste streams contaminated with hydrophobic organic compounds, such as metals [83,84] and polycyclic aromatic hydrocarbons. This results in an improvement in soil quality, which is crucial for the growth of crops. Biosurfactants from the lipid classes of mannosylerythritol, rhamnolipids, and lipopeptides can be utilised as biopesticides to manage a variety of pests, diseases, phytopathogenic fungi, and weeds because of their antimicrobial action [85,86].Regarding the inhibition of the activity of aphids, mosquitos, and harmful toxins produced by the fungus Aspergillus parasiticum in peanut, cotton, and maize crops, other lipopeptides and some glycolipids have also demonstrated promising results in this field, preventing microbial infections and pest infestations [31,74].
Through the effective distribution of metals and micronutrients in the soil and the creation of biofilm on roots, biosurfactants and microorganisms that produce them can both serve as nutrients for plants (a carbon source) and aid in the absorption of essential substances for their proper development. They can also protect plants from harmful substances. [82,87,88]
Conclusion
In the agricultural, cosmetic, food, pharmaceutical, and environmental industries, there is a rising demand for innovative specialised chemicals. It is only natural to go to the microbial world to meet the demand for these chemicals, which must be both effective and environmentally safe. Realising a new chemical product\'s potential is challenging, though, since concerns about economy, efficacy, and efficiency arise. The food and textile industries, environmental cleanup, and the recovery of fossil fuels are all possible applications for biosurfactants. The current lack of widespread use of biosurfactants is a result of the high cost of production brought on by the use of pricey substrates and ineffective recovery techniques. Use of low-cost carbon substrates may have a substantial impact on the economics of biosurfactant manufacturing. In this review, we\'ve covered a full analysis of the numerous ways to make biosurfactants cost-effective, as well as their more recent roles as high-end specialty chemicals, biological pest controllers, and a new breed of molecules for the cosmetic and healthcare industries. Biosurfactants will be successful chemicals in the future when combined with large-scale fermentation, genetic engineering, and metabolic engineering.
References
[1] C.B.B. Farias, F.C.G. Almeida, I.A. Silva, T.C. Souza, H.M. Meira, R. de, C. F. Soares da Silva, J.M. Luna, V.A. Santos, A. Converti, I.M. Banat, L.A. Sarubbo, Production of green surfactants: market prospects, Electron. J. Biotechnol. 51 (2021) 28–39, https://doi.org/10.1016/j.ejbt.2021.02.002. [2] Yin H, Qiang Y, Jia Y, Ye J, Peng H, et al. (2009) Characteristics of biosurfactant produced by Pseudomonas aeruginosa S6 isolated from oil –containing wastewater. Process Biochem 44: 302-308. [3] (Fietcher 1992; Zajic and Stiffens, 1994; Makker and Cameotra, 1998). [4] C.B.B. Farias, F.C.G. Almeida, I.A. Silva, T.C. Souza, H.M. Meira, R. de, C. F. Soares da Silva, J.M. Luna, V.A. Santos, A. Converti, I.M. Banat, L.A. Sarubbo, Production of green surfactants: market prospects, Electron. J. Biotechnol. 51 (2021) 28–39, https://doi.org/10.1016/j.ejbt.2021.02.002. review. Biores Tech, 51: 1-12.) [5] Lotfabad TB, Shourian M, Roostaazad R, Najafabadi AR, Adelzadeh MR, et al. (2009) Colloids Surf B Biointerfaces 69: 183-193. [6] Chirwa EMN, Bezza FA. Petroleum hydrocarbons spill in the environment and abundance of microbial community capable of biosurfactant production. J. Petroleum environment biotechnology. 2015; 6:237. [7] Van Hamme JD, Singh A, Ward OP. Physiolocial aspects, part 1 in a series of paper devoted to surfactants in microbiology and biotechnology. Biotecnnology advance; 2006; 24: 604-620. [8] Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61: 47- 64. [9] Ron EZ, Rosenberg E (2001) Natural roles of biosurfactants. Environ Microbiol 3: 229-236. [10] Adamczak M, Bednarski W (2000) Influence of medium composition and aeration on the synthesis of biosurfactants produced by Candida antartica. Biotechnol Lett 22: 313-316. [11] D.K.F. Santos, R.D. Rufino, J.M. Luna, V.A. Santos, L.A. Sarubbo, Biosurfactants: multifunctional biomolecules of the 21st century, Int. J. Mol. Sci. 17 (2016) 1–31, https://doi.org/10.3390/ijms17030401. [12] C.E. Drakontis, S. Amin, Biosurfactants: formulations, properties, and applications, Curr. Opin. Colloid Interface Sci. 48 (2020) 77–90, https://doi.org/10.1016/j.cocis.2020.03.013. [13] D.K.F. Santos, H.M. Meira, R.D. Rufino, J.M. Luna, L.A. Sarubbo, Biosurfactant production from Candida lipolytica in bioreactor and evaluation of its toxicity for application as a bioremediation agent, Process Biochem. 54 (2017) 20–27, https://doi.org/10.1016/j.procbio.2016.12.020. [14] M.S. Twigg, N. Baccile, I.M. Banat, E. D, eziel, R. Marchant, S. Roelants, I.N.A. Van Bogaert, Microbial biosurfactant research: time to improve the rigour in the reporting of synthesis, functional characterization and process development, Microb. Biotechnol. 14 (2021) 147–170, https://doi.org/10.1111/1751-7915.13704. [15] P.P.F. Brasileiro, D.G. De Almeida, J.M. De Luna, R.D. Rufino, V.A. Dos Santos, L. A. Sarubbo, Optimization of biosurfactant production from Candida guiliermondii using a rotate central composed design, Chem. Eng. Trans. 43 (2015) 1411–1416, https://doi.org/10.3303/CET1543236. [16] A.P. Kumar, A. Janardhan, S. Radha, B. Viswanath, G. Narasimha, Statistical approach to optimize production of biosurfactant by Pseudomonas aeruginosa 2297, 3 Biotech 5 (2015) 71–79, https://doi.org/10.1007/s13205-014-0203-3. [17] T.G. Ambaye, M. Vaccari, S. Prasad, S. Rtimi, Preparation, characterization and application of biosurfactant in various industries: a critical review on progress, challenges and perspectives, Environ. Technol. Innov. 24 (2021), 102090, https://doi.org/10.1016/j.eti.2021.102090. [18] Rahman PKSM, Gakpe E (2008) Production, characterization and application of Biosurfactants-Review. Biotechnology 7: 360-370. [19] Zinjarde and Pant investigated how pH affected the amount of biosurfactant generated. [20] Adamczak M, Bednarski W (2000) Influence of medium composition and aeration on the synthesis of biosurfactants produced by Candida antartica. Biotechnol Lett 22: 313-316. [21] Zinjarde SS, Pant A (2002) Emulsifier from a tropical marine yeast Yarrowialipolytica NCIM 3589. J Basic Microbiol 42: 67-73. [22] Mulugeta, K., Kamaraj, M., Tafesse, M., & Aravind, J. (2021). A review on production, properties, and applications of microbial surfactants as a promising biomolecule for environmental applications. Strategies and tools for pollutant mitigation: avenues to a cleaner environment, 3-28. [23] de Andrade Bustamante, R., de Oliveira, J. S., & Dos Santos, B. F. (2023). Modeling biosurfactant production from agroindustrial residues by neural networks and polynomial models adjusted by particle swarm optimization. Environmental Science and Pollution Research, 30(3), 6466-6491. [24] Sivapathasekaran, C., & Sen, R. (2017). Origin, properties, production and purification of microbial surfactants as molecules with immense commercial potential. Tenside Surfactants Detergents, 54(2), 92-107. [25] Caputi, L., Franke, J., Farrow, S. C., Chung, K., Payne, R. M., Nguyen, T. D., ... & O’Connor, S. E. (2018). Missing enzymes in the biosynthesis of the anticancer drug vinblastine in Madagascar periwinkle. Science, 360(6394), 1235-1239. [26] Hassan, M., Ding, W., Shi, Z., & Zhao, S. (2016). Methane enhancement through co-digestion of chicken manure and thermo-oxidative cleaved wheat straw with waste activated sludge: AC/N optimization case. Bioresource technology, 211, 534-541. [27] Ismail, W., Shammary, S.A., El-Sayed, W.S., Obuekwe, C., El Nayal, A.M., Raheem, A.S.A. and Al-Humam, A. (2015) Stimulation of rhamnolipid biosurfactants production in Pseudomonas aeruginosa AK6U by organosulfur compounds provided as sulfur sources. Biotechnol Rep 7, 55–63. [28] Biniarz, P., ?ukaszewicz, M. and Janek, T. (2017) Screening concepts, characterization and structural analysis of microbial-derived bioactive lipopeptides: a review. Crit Rev Biotechnol 37, 393–410. [29] Cruz, J.M., Hughes, C., Quilty, B., Montagnolli, R.N. and Bidoia, E.D. (2017) Agricultural feedstock supplemented with manganese for biosurfactant production by Bacillus subtilis. Waste Biomass Valori 9, 613–618. [30] W.H. Haider, Estimates of total oil & gas reserves in the world, future of oil and gas companies and SMART investments by E & P companies in renewable energy sources for future energy needs, in: Int. Pet. Technol. Conf., IPTC, Dhahran, Kingdom of Saudi Arabia, 2020, https://doi.org/10.2523/IPTC-19729-MS. [31] E.O. Fenibo, G.N. Ijoma, R. Selvarajan, C.B. Chikere, Microbial surfactants: The next generation multifunctional biomolecules for applications in the petroleum industry and its associated environmental remediation, Microorganisms 7 (2019) 1–29, https://doi.org/10.3390/microorganisms7110581. [32] D.K.F. Santos, R.D. Rufino, J.M. Luna, V.A. Santos, L.A. Sarubbo, Biosurfactants: multifunctional biomolecules of the 21st century, Int. J. Mol. Sci. 17 (2016) 1–31, https://doi.org/10.3390/ijms17030401. [33] A.O. Gbadamosi, R. Junin, M.A. Manan, A. Agi, A.S. Yusuff, An Overview of Chemical Enhanced oil Recovery: Recent Advances and Prospects, Springer Berlin Heidelberg, 2019, https://doi.org/10.1007/s40089-019-0272-8. [34] S.J. Geetha, I.M. Banat, S.J. Joshi, Biosurfactants: production and potential applications in microbial enhanced oil recovery (MEOR), Biocatal. Agric. Biotechnol. 14 (2018) 23–32, https://doi.org/10.1016/j.bcab.2018.01.010. [35] D.G. de Almeida, R. de, C.F. Soares Da Silva, J.M. Luna, R.D. Rufino, V.A. Santos, I.M. Banat, L.A. Sarubbo, Biosurfactants: promising molecules for petroleum biotechnology advances, Front. Microbiol. 7 (2016) 1–14, https://doi.org/ 10.3389/fmicb.2016.01718. [36] F. Shakeri, H. Babavalian, M.A. Amoozegar, Z. Ahmadzadeh, S. Zuhuriyanizadi, M.P. Afsharian, Production and application of biosurfactants in biotechnology, Biointerface Res. Appl. Chem. 11 (2020) 10446–10460, https://doi.org/ 10.33263/BRIAC113.1044610460. [37] J.F.B. Pereira, E.J. Gudina, ˜ R. Costa, R. Vitorino, J.A. Teixeira, J.A.P. Coutinho, L. R. Rodrigues, Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications, Fuel 111 (2013) 259–268, https://doi.org/10.1016/j.fuel.2013.04.040. [38] H. Alkan, S. Mukherjee, F. Kogler, ¨ Reservoir engineering of in-situ MEOR; impact of microbial community, J. Pet. Sci. Eng. 195 (2020), https://doi.org/10.1016/j. petrol.2020.107928. [39] N. Yamashita, K. Yokoyama, S. Kawashima, H. Yamaguchi, T. Sato, Organic nutrients for microbial enhanced oil recovery, Pet. Sci. Technol. 36 (2018) 1991–1997, https://doi.org/10.1080/10916466.2018.1528273. [40] G. Sun, J. Hu, Z. Wang, X. Li, W. Wang, Dynamic investigation of microbial activity in microbial enhanced oil recovery (MEOR), Pet. Sci. Technol. 36 (2018) 1265–1271, https://doi.org/10.1080/10916466.2018.1468776. [41] G. Rawat, A. Dhasmana, V. Kumar, Biosurfactants: the next generation biomolecules for diverse applications, Environ. Sustain. 3 (2020) 353–369, https://doi.org/10.1007/s42398-020-00128-8. [42] N. Khondee, S. Tathong, O. Pinyakong, R. Müller, S. Soonglerdsongpha, C. Ruangchainikom, C. Tongcumpou, E. Luepromchai, Lipopeptide biosurfactant production by chitosan-immobilized Bacillus sp. GY19 and their recovery by foam fractionation, Biochem. Eng. J. 93 (2015) 47–54, https://doi.org/10.1016/j. bej.2014.09.001. [43] V.M. Alvarez, D. Jurelevicius, J.M. Marques, P.M. de Souza, L.V. de Araújo, T. G. Barros, R.O.M.A. de Souza, D.M.G. Freire, L. Seldin, Bacillus amyloliquefaciens TSBSO 3.8, a biosurfactant-producing strain with biotechnological potential for microbial enhanced oil recovery, Colloids Surf. B Biointerfaces 136 (2015) 14–21, https://doi.org/10.1016/j.colsurfb.2015.08.046. [44] J.M.D.A. Camara, ˆ M.A.S.B. Sousa, E.L. Barros Neto, M.C.A. Oliveira, Application of rhamnolipid biosurfactant produced by Pseudomonas aeruginosa in microbialenhanced oil recovery (MEOR), J. Pet. Explor. Prod. Technol. 9 (2019) 2333–2341, https://doi.org/10.1007/s13202-019-0633-x. [45] E.J. Gudina, ˜ J.F.B. Pereira, R. Costa, J.A.P. Coutinho, J.A. Teixeira, L. R. Rodrigues, Biosurfactant-producing and oil-degrading Bacillus subtilis strains enhance oil recovery in laboratory sand-pack columns, J. Hazard. Mater. 261 (2013) 106–113, https://doi.org/10.1016/j.jhazmat.2013.06.071. [46] R. Silva, D. Almeida, R. Rufino, J. Luna, V. Santos, L. Sarubbo, Applications of biosurfactants in the petroleum industry and the remediation of oil spills, Int. J. Mol. Sci. 15 (2014) 12523–12542, https://doi.org/10.3390/ijms150712523. [47] R. Chandankere, J. Yao, M.M.F. Choi, K. Masakorala, Y. Chan, An efficient biosurfactant-producing and crude-oil emulsifying bacterium Bacillus methylotrophicusUSTBa isolated from petroleum reservoir, Biochem. Eng. J. 74 (2013) 46–53, https://doi.org/10.1016/j.bej.2013.02.018. [48] C. Zou, M. Wang, Y. Xing, G. Lan, T. Ge, X. Yan, T. Gu, Characterization and optimization of biosurfactants produced by Acinetobacter baylyi ZJ2 isolated from crude oil-contaminated soil sample toward microbial enhanced oil recovery applications, Biochem. Eng. J. 90 (2014) 49–58, https://doi.org/10.1016/j. bej.2014.05.007. [49] I.G.S. da Silva, F.C.G. de Almeida, N.M.P. da Rocha e Silva, A.A. Casazza, A. Converti, L.A. Sarubbo, Soil bioremediation: overview of technologies and trends, Energies 13 (2020), https://doi.org/10.3390/en13184664. [50] I.J.B. Durval, A.H.R. Mendonça, I.V. Rocha, J.M. Luna, R.D. Rufino, A. Converti, L.A. Sarubbo, Production, characterization, evaluation and toxicity assessment of a Bacillus cereus UCP 1615 biosurfactant for marine oil spills bioremediation, Mar. Pollut. Bull. 157 (2020), 111357, https://doi.org/10.1016/j. marpolbul.2020.111357. [51] V.V. Jadhav, A. Yadav, Y.S. Shouche, S. Aphale, A. Moghe, S. Pillai, A. Arora, R. K. Bhadekar, Studies on biosurfactant from Oceanobacillus sp. BRI 10 isolated from Antarctic sea water, Desalination 318 (2013) 64–71, https://doi.org/ 10.1016/j.desal.2013.03.017. [52] F.E. Mouafi, M.M. Abo Elsoud, M.E. Moharam, Optimization of biosurfactant production by Bacillus brevis using response surface methodology, Biotechnol. Rep. 9 (2016) 31–37, https://doi.org/10.1016/j.btre.2015.12.003. [53] J.M. Luna, R.D. Rufino, A.M.A.T. Jara, P.P.F. Brasileiro, L.A. Sarubbo, Environmental applications of the biosurfactant produced by Candida sphaerica cultivated in low-cost substrates, Colloids Surf. A Physicochem. Eng. Asp. 480 (2015) 413–418, https://doi.org/10.1016/j.colsurfa.2014.12.014. [54] R. Khademolhosseini, A. Jafari, S.M. Mousavi, H. Hajfarajollah, K.A. Noghabi, M. Manteghian, Physicochemical characterization and optimization of glycolipid biosurfactant production by a native strain of Pseudomonas aeruginosa HAK01 and its performance evaluation for the MEOR process, RSC Adv. 9 (2019) 7932–7947, https://doi.org/10.1039/C8RA10087J. [55] M.J. Chaprao, ˜ R. de, C.F.S. da Silva, R.D. Rufino, J.M. Luna, V.A. Santos, L. A. Sarubbo, Production of a biosurfactant from Bacillus methylotrophicus UCP1616 for use in the bioremediation of oil-contaminated environments, Ecotoxicology 27 (2018) 1310–1322, https://doi.org/10.1007/s10646-018- 1982-9. [56] P.L. Fernandes, E.M. Rodrigues, F.R. Paiva, B.A.L. Ayupe, M.J. McInerney, M. R. Totola, ´ Biosurfactant, solvents and polymer production by Bacillus subtilis RI4914 and their application for enhanced oil recovery, Fuel 180 (2016) 551–557, https://doi.org/10.1016/j.fuel.2016.04.080. [57] T. Minucelli, R.M. Ribeiro-Viana, D. Borsato, G. Andrade, M.V.T. Cely, M.R. de Oliveira, C. Baldo, M.A.P.C. Celligoi, Sophorolipids production by candida bombicola ATCC 22214 and its potential application in soil bioremediation, Waste Biomass Valoriz. 8 (2017) 743–753, https://doi.org/10.1007/s12649-016- 9592-3. [58] D. Hentati, A. Chebbi, A. Mahmoudi, F. Hadrich, M. Cheffi, I. Frikha, S. Sayadi, M. Chamkha, Biodegradation of hydrocarbons and biosurfactants production by a newly halotolerant Pseudomonas sp. strain isolated from contaminated seawater, Biochem. Eng. J. 166 (2021), 107861, https://doi.org/10.1016/j. bej.2020.107861. [59] M.R. Chirani, E. Kowsari, T. Teymourian, S. Ramakrishna, Environmental impact of increased soap consumption during COVID-19 pandemic: biodegradable soap production and sustainable packaging, Sci. Total Environ. 796 (2021), https:// doi.org/10.1016/j.scitotenv.2021.149013. [60] A. Çelik, E.B. Manga, A. Çabuk, I.M. Banat, Biosurfactants’ potential role in combating covid-19 and similar future microbial threats p?nar, Appl. Sci. 11 (2021) 1–16, https://doi.org/10.3390/app11010334. [61] C.B.B. Farias, F.C.G. Almeida, I.A. Silva, T.C. Souza, H.M. Meira, R. de, C. F. Soares da Silva, J.M. Luna, V.A. Santos, A. Converti, I.M. Banat, L.A. Sarubbo, Production of green surfactants: market prospects, Electron. J. Biotechnol. 51 (2021) 28–39, https://doi.org/10.1016/j.ejbt.2021.02.002. [62] A. Perfumo, I.M. Banat, R. Marchant, Going green and cold: biosurfactants from low-temperature environments to biotechnology applications, Trends Biotechnol. 36 (2018) (2018) 277–289, https://doi.org/10.1016/j.tibtech.2017.10.016. [63] S. Nadaf, V.M. Kumbar, S. Killedar, A.I. Torvi, J.H. Hoskeri, A.K. Shettar, Microbial biosurfactants as cleaning and washing agents. Microbial Biosurfactants, Springer, 2021, pp. 293–314, https://doi.org/10.1007/978-981- 15-6607-3_14. [64] A.A. Jimoh, J. Lin, Biosurfactant: a new frontier for greener technology and environmental sustainability, Ecotoxicol. Environ. Saf. 184 (2019), 109607, https://doi.org/10.1016/j.ecoenv.2019.109607. [65] M. Bouassida, N. Fourati, I. Ghazala, S. Ellouze-Chaabouni, D. Ghribi, Potential application of Bacillus subtilis SPB1 biosurfactants in laundry detergent formulations: Compatibility study with detergent ingredients and washing performance, Eng. Life Sci. 18 (2018) 70–77, https://doi.org/10.1002/ elsc.201700152. [66] D. Fei, G.W. Zhou, Z.Q. Yu, H.Z. Gang, J.F. Liu, S.Z. Yang, R.Q. Ye, B.Z. Mu, Lowtoxic and nonirritant biosurfactant surfactin and its performances in detergent formulations, J. Surfactants Deterg. 23 (2020) 109–118, https://doi.org/ 10.1002/jsde.12356. [67] C.E. Drakontis, S. Amin, Design of sustainable lip gloss formulation with biosurfactants and silica particles, Int. J. Cosmet. Sci. 42 (2020) 573–580, https:// doi.org/10.1111/ics.12642. [68] Cosmetics Europe, Socio-Economic Contribution of the European Cosmetics Industry, 2019. ?https://www.cosmeticseurope.eu/files/4715/6023/8405/Socio -Economic_Contribution_of_the_European_Cosmetics_Industry_Report_2019.pdf? (Accessed 2 February 2021). [69] I.O. Olasanmi, R.W. Thring, The role of biosurfactants in the continued drive for environmental sustainability, Sustain 10 (2018) 1–12, https://doi.org/10.3390/ su10124817. [70] S. Akbari, N.H. Abdurahman, R.M. Yunus, F. Fayaz, O.R. Alara, Biosurfactants—a new frontier for social and environmental safety: a mini review, Biotechnol. Res. Innov. 2 (2018) 81–90, https://doi.org/10.1016/j.biori.2018.09.001. [71] U.B. Bolmal, R.P. Subhod, A.P. Gadad, A.S. Patil, Formulation and evaluation of carbamazepine tablets using biosurfactant in ternary solid dispersion system, Indian J. Pharm. Educ. Res. 54 (2020) 302–309, https://doi.org/10.5530/ ijper.54.2.35. [72] S.A. Adu, P.J. Naughton, R. Marchant, I.M. Banat, Microbial biosurfactants in cosmetic and personal skincare pharmaceutical formulations, Pharmaceutics 12 (2020) 1–21, https://doi.org/10.3390/pharmaceutics12111099. [73] D.L. Gutnick, H. Bach, Biosurfactants. Comprehensive Biotechnology, Elsevier, 2017, pp. 731–757, https://doi.org/10.1016/B978-0-12-809633-8.09184-6. [74] G. Rawat, A. Dhasmana, V. Kumar, Biosurfactants: the next generation biomolecules for diverse applications, Environ. Sustain. 3 (2020) 353–369, https://doi.org/10.1007/s42398-020-00128-8. [75] A.R. Markande, D. Patel, S. Varjani, A review on biosurfactants: properties, applications and current developments, Bioresour. Technol. 330 (2021), 124963, https://doi.org/10.1016/j.biortech.2021.124963. [76] C.E. Drakontis, S. Amin, Biosurfactants: formulations, properties, and applications, Curr. Opin. Colloid Interface Sci. 48 (2020) 77–90, https://doi.org/ 10.1016/j.cocis.2020.03.013. [77] S.S. Giri, Application of microbial biosurfactants in the pharmaceutical industry, in: Microb. Biosurfactants. Environ. Microb. Biotechnol., Springer, Singapore, 2021, pp. 251–269, https://doi.org/10.1007/978-981-15-6607-3_12. [78] N. Jemil, H. Ben Ayed, A. Manresa, M. Nasri, N. Hmidet, Antioxidant properties, antimicrobial and anti-adhesive activities of DCS1 lipopeptides from Bacillus methylotrophicus DCS1, BMC Microbiol 17 (2017) 144, https://doi.org/10.1186/ s12866-017-1050-2. [79] G.A. P?aza, J. Chojniak, I.M. Banat, Biosurfactant mediated biosynthesis of selected metallic nanoparticles, Int. J. Mol. Sci. 15 (2014) 13720–13737, https:// doi.org/10.3390/ijms150813720. [80] M. Elshikh, S. Funston, A. Chebbi, S. Ahmed, R. Marchant, I.M. Banat, Rhamnolipids from non-pathogenic Burkholderiathailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens, N. Biotechnol. 36 (2017) 26–36, https://doi.org/ 10.1016/j.nbt.2016.12.009. [81] D.P. Sachdev, S.S. Cameotra, Biosurfactants in agriculture, Appl. Microbiol. Biotechnol. 97 (2013) 1005–1016, https://doi.org/10.1007/s00253-012-4641-8. [82] J. Kohl, ¨ R. Kolnaar, W.J. Ravensberg, Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy, Front. Plant Sci. 10 (2019) 1–19, https://doi.org/10.3389/fpls.2019.00845. [83] A. Ravindran, A. Sajayan, G.B. Priyadharshini, J. Selvin, G.S. Kiran, Revealing the efficacy of thermostable biosurfactant in heavy metal bioremediation and surface treatment in vegetables, Front. Microbiol. 11 (2020) 1–11, https://doi.org/ 10.3389/fmicb.2020.00222. [84] A. Franzetti, I. Gandolfi, L. Fracchia, J. Van Hamme, P. Gkorezis, R. Marchant, I. Banat, Biosurfactant use in heavy metal removal from industrial effluents and contaminated sites, in: Biosurfactants, CRC Press, 2014, pp. 361–370, https://doi. org/10.1201/b17599-20. [85] L. Rodrigues, I.M. Banat, J. Teixeira, R. Oliveira, Biosurfactants: Potential applications in medicine, J. Antimicrob. Chemother. 57 (2006) 609–618, https:// doi.org/10.1093/jac/dkl024. [86] F.J. Ochoa-Loza, J.F. Artiola, R.M. Maier, Stability constants for the complexation of various metals with a rhamnolipid biosurfactant, J. Environ. Qual. 30 (2001) 479–485, https://doi.org/10.2134/jeq2001.302479x. [87] R. Singh, B.R. Glick, D. Rathore, Biosurfactants as a biological tool to increase micronutrient availability in soil: a review, Pedosphere 28 (2018) 170–189, https://doi.org/10.1016/S1002-0160(18)60018-9. [88] H.W.C. Araújo, R.F.S. Andrade, D. Montero-Rodríguez, D. Rubio-Ribeaux, C. A. Alves Da Silva, G.M. Campos-Takaki, Sustainable biosurfactant produced by Serratia marcescens UCP 1549 and its suitability for agricultural and marine bioremediation applications, Microb. Cell Fact. 18 (2019) 1–13, https://doi.org/ 10.1186/s12934-018-1046-0. [89] Yin H, Qiang Y, Jia Y, Ye J, Peng H, et al. (2009) Characteristics of biosurfactant produced by Pseudomonas aeruginosa S6 isolated from oil –containing wastewater. Process Biochem 44: 302-308. [90] Makkar RS, Rockne KJ (2003) Comparison of synthetic surfactants and biosurfactants in enhancing biodegradation of polycyclic aromatic hydrocarbon. Environ Toxicol Chem 22: 2280-2292.
Copyright
Copyright © 2023 Mr. Gopani Juvin Shaileshbhai, Ms. Trupti Pandya. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET54727
Publish Date : 2023-07-10
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

