Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Physico-Chemical and Microbiological Analysis of Groundwater in Bathindi, Jammu, (J&K)
Authors: Nahira Shameem, Sakshi Sharma
DOI Link: https://doi.org/10.22214/ijraset.2024.65580
Certificate: View Certificate
Abstract
This Paper has been made in order to examine the “Physico-chemical and microbiological analysis of groundwater in Bathindi, Jammu, (J&K)”. Water is life’s matter and matrix, mother and medium (Saxena and Saxena, 2015). Water is a key component in determining the quality of our lives. Although water covers about 70 percent of the earth’s total surface, only 0.3% of it can be used by humans (Sabo and Christopher, 2014; Tiimub et al., 2012). Water is one of the basic needs of life and essential for survival (Das et al., 2014). Water is a vital component in socio-economic life of people (Shankar et al., 2014). The present analytical study of water samples evinces that the quality of water vis-à-vis various physico-chemical and biological parameters are within the permissible limits except for the parameters like TDS, Calcium and Total Hardness which have exceeded the desirable limits compared with National and international standards. According to standards recommended by British Ministry of Health (1957), the water quality comes under the category of satisfactory except for 2nd and 4th (Tubewell, Home and Tap), 1st, and 6th (Home and Tap), 7th (Tubewell) and 8th (Tubewell and Tap) readings. The total coliform count observed also exceeded the permissible limits, indicates that there is entry of sewage and soil sediments in the distribution pipes.
Introduction
I. INTRODUCTION
Groundwater is an important source of drinking water for mankind. It contains over 90% of fresh water resources, is an important reserve of good quality water and it is also used for agricultural, industrial, household, recreational and environmental activities all over the world. The quality of ground water varies from place to place, with the depth of water table and from season to season and is primarily governed by the extent and composition of dissolved solids present in it. In recent years, an increasing threat to ground water quality due to human activities has become of great importance. The adverse effects on ground water quality are the results of man’s activity at ground surface, unintentionally by agriculture, domestic and industrial effluents, sub-surface and surface disposal of sewage and industrial wastes. The quality of ground water is of great importance in determining the suitability of particular ground water for a certain use in order to evaluate water quality, the present study was undertaken to get information on the ground water quality and its distribution by analyzing various physico-chemical characteristics viz. temperature, total dissolved solids, pH, electrical conductivity, dissolved oxygen, free carbon dioxide, carbonate, bicarbonate, chloride, calcium, magnesium, total hardness, biochemical oxygen demand and microbiological parameter i.e. MPN index per 100ml., at Narwal pumping station and its two distribution points in Bathindi area, for which there is no published record till date.
A. Objectives
- To examine the Bi-monthly variations in air and water temperature, EC and TDS, pH and CO2, D.O. and B.O.D., HCO3- and Cl- Ca, Mg and TH of different sites.
- To analyze the Comparison of water quality of Tube well, Tap (home) and Tap (lane) water with National and International standards.
- To Understand the Coefficient correlation between physico-chemical parameters of Tap (home) water samples.
II. METHODOLOGY
A. Sampling and Analysis of Water
1) Physico-chemical Analysis
Bi-monthly, water samples from the two sampling sites were collected in the plastic bottles. Before the collection of water samples, physical factor like air and water temperature was recorded. The methods used for analysis of various parameters of water viz. temperature, total dissolved solids, pH, electrical conductivity, dissolved oxygen, free carbon dioxide, carbonate, bicarbonate, chloride, calcium, magnesium, total hardness and biological oxygen demand are discussed as follows:
- Temperature: The air and water temperature was recorded by using a centigrade mercury bulb thermometer. Air temperature was recorded by keeping thermometer in shade while water temperature was recorded by dipping thermometer in the water sample.
- TDS: TDS was determined using Water / Soil Analyzer Kit Model Century CMK 731.
- Electrical Conductivity: Electrical conductivity was determined by using Water / Soil Analyzer Kit Model Century CMK 731.
- pH: pH was determined by Water / Soil Analyzer Kit Model Century CMK 731.
- Dissolved Oxygen: Dissolved oxygen was measured by Alsterberg's alkaline sodium azide method (APHA, 1998).
- Free CO2: Free CO2 is estimated by the titrimetric method recommended by APHA (1998).
- Bicarbonate: Bicarbonate was estimated by titrimetric method, using methyl orange as indicator and sulphuric acid as titrant (APHA, 1998).
- BOD (Biological Oxygen Demand): The BOD was measured by 5-day's BOD method (APHA, 1998).
- Chloride: Chloride was determined by Argentometric method (APHA, 1998).
- Calcium: Calcium was determined by disodium varsenate method, using murexide indicator (APHA, 1998).
- Magnesium and Total hardness: Magnesium and total hardness were estimated by EDTA titrimetric method using Erichrome Black-T indicator (APHA, 1998).
2) Microbiological Analysis
Bi-monthly, water samples from two sampling sites were collected in the pre-sterilized BOD bottles. For microbiological studies, quality of water was determined using most probable number (MPN) method. The MPN method was used to determine the presence of gas producing lactose fermenters and most probable number of coliforms present in 100 ml of water. The standard multiple tube dilution technique was used for detection of total coliforms by inoculation of samples into tubes of MacConkey broth and incubation at 37°C for 48 h.
- Co-efficient of Correlation: Co-efficient Matrix and Co-efficient of correlation (r) of MPN index per 100 ml., with various physico-chemical parameters of water, was calculated, for each site, by using the Microsoft Excel.
- Water Quality Index: was calculated for assessing the suitability of ground water for drinking purposes considering nine important physico-chemical parameters viz. pH, alkalinity, total hardness, dissolved oxygen, B.O.D., chloride, calcium, magnesium, electrical conductivity from the following equation:
WQI = 9∑n=1 qn.Wn
where, Wn = Unit weight
qn = Quality rating
WQI = Water Quality Index
III. STUDY AREA
J&K State is located in the northern part of India. In this study, Narwal-Bathindi area of Jammu district area is selected. The Jammu district is located at 32° 44´ N Latitude and 74° 52´ E Longitude, at an altitude of 753 feet, above mean sea level. The study was conducted from Feb to May. Total 24 samples from 3 different locations were collected, 8 from each site. 8 samples were taken from Narwal pumping station and 16 samples were taken from two distribution point one at home and other at tap in lane in Bathindi area.
Fig. 1: Satellite image of the Tube well, home and Tap (lane) water samples collection sites.
IV. RESULTS AND DISCUSSION
The results of physico-chemical characteristics of three sampling sites have been tabulated in Tables 1-3 and depicted in Figs. 8-13 .
A. SITE I: Pumping station water sample
1) Temperature
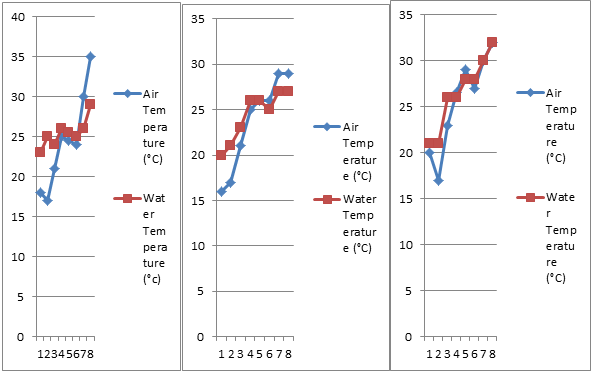
Air temperature varied from 17°C (20/02/18) to 35°C (23/5/18). Lowest value is observed in the month of February and highest in May of present investigation (Table 1 and Fig.8).
Water temperature ranged between 23°C (5/02/18) and 29°C (23/5/18). Thermostatic characteristics of ground water may explain narrow variation in water temperature. Increased solar radiation due to comparatively longer day length, may explain May rise in air and water temperature (Khajuria and Dutta, 2011). The difference in temperature is due to the seasonal variations and is in accordance with the findings of Parihar et al. (2012).
2) pH
An observation of Table 1 and Fig. 10 reveals that pH varied between 6.66 (5/02/18) and 7.21 (7/03/18). The present range is in accordance with the findings of Sehar et al. (2010) and Das et al. (2014). A narrow variation in pH is due to continuous presence of free carbon dioxide and high concentration of bicarbonates (Khajuria and Dutta, 2011).
3) Electrical Conductivity
Electrical conductivity (Table 1 and Fig.9 ) ranged from 0.265 mS/cm (5/02/18) to 0.704 mS/cm (22/3/18). March increase in electrical conductivity may be attributed to the rains. Chemostatic characteristics of ground water may explain a narrow difference of electrical conductivity record and is in accordance with the findings of Reza and Singh (2009) and Khajuria and Dutta (2011).
4) Total Dissolved Solid
A look at the Table 1 and Fig.9 reveals that TDS varied between 0.133mg/l (5/2/18) – 0.354mg/l (22/03/18) and the water is suitable for drinking purposes. Lowest value is observed in the month of February and highest in March.
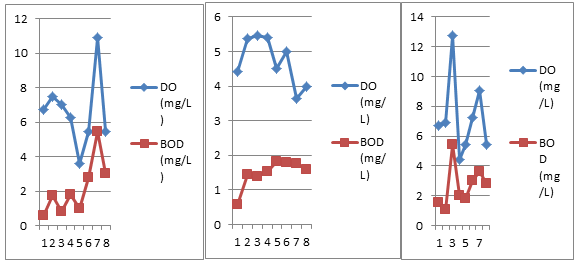
5) Dissolved Oxygen
Dissolved oxygen remained fairly present throughout the study period and has shown variation from 3.63 mg/l (08/05/18) to 5.45 mg/l (7/03/18). Its highest record is in the month of March and lowest value in the month of May (Table 1 and Fig.11). This type of observation has also been made earlier by Khajuria and Dutta (2010b), Sehar et al. (2011) and Patel et al. (2017). The variation in the dissolved oxygen is due to aeration of water during pumping, variable records of temperature, free carbon dioxide, dissolved solids, microbes and decomposition of organic matter (Khajuria and Dutta, 2010b).
6) Biochemical Oxygen Demand
An observation of the Table 1 and Fig.11 reveals that BOD ranged between 0.57mg/l (5/02/18) and 1.81mg/l (6/04/18) and is in accordance with the findings of Khajuria and Dutta (2010b); Bundela et al. (2012). A good amount of dissolved oxygen in water and good filtration of water by rocks in the catchment has correlation with variable records of BOD in the ground water (Khajuria and Dutta, 2010b).
7) Free Carbon Dioxide
Free carbon dioxide (Table 1 and Fig. 10) varied from 8.85mg/l (20/02/18) to 44.27mg/l (5/02/18 and 6/04/18) and is in agreement with the findings of Khajuria and Dutta (2011) and Ankush (2012). Agitation of water during its pumping from ground through pipes lead to loss of free carbon dioxide and also responsible for the carbonate absence (Khajuria and Dutta, 2011).
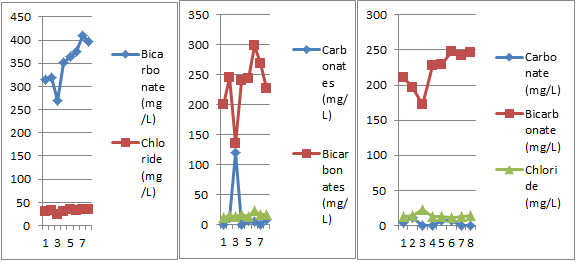
8) Bicarbonate
A look at the Table 1 and Fig.12 reveals that bicarbonate varied between 269.85mg/l (7/03/18) and 409.53mg/l (8/05/18) and is in accordance with the findings of Joarder et al. (2008) and Khan and Rehman (2017). Its highest record is in the month of February and lowest value in the month of April. Variations in bicarbonate in ground water may be due to variable records of bicarbonate minerals in rocks and soil and their dissolution in water, carbon dioxide and organic matter in the vicinity and its decomposition (Khajuria and Dutta, 2011).
9) Chloride
Chloride ranged between 24.6mg/l (7/03/18) and 35.26mg/l (6/04/18 and 23/05/18) and is in agreement with the findings of Reza and Singh (2009) and Saleem et al. (2016). Its highest record is in the month of April and May and lowest value in the month of March (Table 1 and Fig.12). The concentration of chloride in water samples is generally due to the low deposits of chloride in rocks in catchment area, variable deposits of organic matter in the soil and sewage infiltration (Khajuria and Dutta, 2011); domestic waste, poor sanitary conditions and leaching (Mostafa et al., 2017).
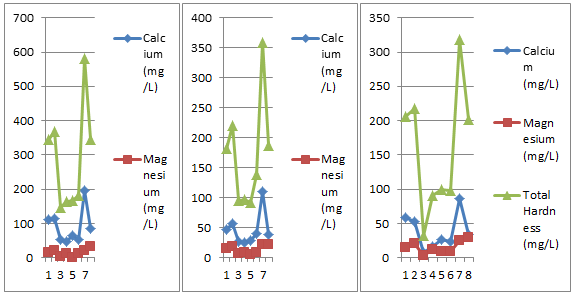
10) Calcium, Magnesium and Total Hardness
An observation of the Table 1 and Fig.13 reveals that calcium, magnesium and total hardness fluctuated between 47.29mg/l (22/03/18) to 197.17mg/l (8/05/18), 0.52mg/l (6/04/18) to 31.45mg/l (23/05/18) and 144.84mg/l (7/03/18) to 580.5mg/l (8/05/18), respectively and are in accordance with the findings of Yadav et al. (2012). Their highest record is in the month of May and lowest value in the month of March and April, respectively. This variation may be coincided with nature and weathering of rocks in the catchment and water depth, variable records of free carbon dioxide, variable deposits of organic matter in the soil and sewage infiltration (Khajuria and Dutta, 2011).
B. Site II: Home Tap water sample
1) Temperature
Air temperature varied between 16°C (5/2/18) to 29°C (8/05/18 and 23/05/18). Lowest value is observed in the month of February and highest in May. Water temperature ranged between 20°C (5/02/18) and 27°C (8/05/18 and 23/05/18). Lowest value is observed in the month of February and highest in May (Table 2 and Fig.8 ). The difference in temperature is due to heating and cooling of pipes, agitation of water in pipes during supply (Khajuria and Dutta, 2011) and the seasonal variations (Parihar et al., 2012).
2) pH
A look at Table 2 and Fig.10 reveals the variations in pH from 7.15 (5/02/18) - 7.68 (7/03/18) and is in agreement with the findings of Das et al. (2014) and Patel et al. (2016). This variation may be due to variation in free carbon dioxide, dissolved oxygen, caused during agitation of water in pipes. An inverse relationship of pH with free carbon dioxide and direct with dissolved oxygen is already on record (Khajuria and Dutta, 2011). As any alteration in water pH is accompanied by the change in other physico-chemical parameters (Parihar et al., 2012).
3) Electrical Conductivity
Electrical conductivity (Table 2 and Fig.9) varied between 0.266 mS/cm (5/02/18) to 0.537 mS/cm (23/04/18) and is less as compared to the findings of Sundar and Saseetharan (2008) and Khajuria and Dutta (2010c). Variable records of mineral constituents like bicarbonate, chloride, calcium, magnesium, total hardness, etc. may explain variations in EC. The variable rate of infiltration of domestic waste is known to increase electrical conductivity (Khajuria and Dutta, 2011).
4) Total Dissolved Solid
TDS ranged between 0.21 mg/l (8/05/18) and 169 mg/l (23/05/18). Lowest value is observed in the month of February and highest in May (Table 2 and Fig.9 ). The higher value is due to the presence of salts in high amount, because of high rate of leaching during the rainy day. This observation is high as compared to the findings of Mishra and Bhatt (2008) and low than the observations made by Parihar et al. (2012).
5) Dissolved Oxygen
Dissolved oxygen (Table 2 and Fig.11 ) fluctuated from 3.63 mg/l (6/04/18) to 10.9 mg/l (8/05/18). These values are in accordance with the findings of Usharani et al. (2010) and higher than the findings of Sehar et al. (2011). Dissolved oxygen reflects the physical and biological processes prevailing in the water (Sehar et al., 2011). The variation in the dissolved oxygen is due to the growth of biofilms inside the pipes, dead ends causing oxygen depletion (Khajuria and Dutta, 2010b).
6) Biochemical Oxygen Demand
During the present study, BOD varied from 0.57 mg/l (5/02/18) to 5.45 mg/l (8/05/18) (Table 2 and Fig.11) and is in accordance with the findings of Usharani et al. (2010) and Khajuria and Dutta (2010b). Growth of algae at breakage points of pipes and sewage contamination during crossing pipes underground or through drains lead to increase in BOD values in the water (Khajuria and Dutta, 2010b).
7) Free Carbon Dioxide
An observation of Table 2 and Fig.10 reveals that free carbon dioxide was present in only 3 samples (5/02/18, 22/03/18 & 8/05/18) and varied between 8.85 (5/02/18) to 17.7mg/l (22/03/18 and 8/05/18). Lowest value is observed in the month of February and highest in March and May. The values are less in compared to the findings of Khajuria and Dutta (2011) and Ankush (2012). Well marked variations in free carbon dioxide in tap water may be due to agitation of water during its flow through pipes, deposits of biofilms inside the pipes and entry of organic matter along with soil sediments and sewage through pipes during crossing of pipes in the drains, etc. and its microbial decomposition Khajuria and Dutta (2011).
8) Carbonate
Carbonate (Table 2 & Fig.12) was present in 5 samples (20/02/18, 7/03/18, 6/04/18, 23/04/18 & 23/05/18) and ranged between 5.35mg/L (23/04/18) and 120.65mg/L (7/03/18). Carbonate remains generally absent in groundwater but its presence in consumer points may be due to the entry of sewage, sediments etc in the pipes through back siphonage, cracks, dislocation, defective joints breakage etc.
9) Bicarbonate
An observation of the Table 2 and Fig.12 reveals that bicarbonate varied between 134.92 mg/l (7/3/18) and 299.33 mg/l (23/4/18) and is in accordance with the study of Joarder et al. (2008) and Khan and Rehman (2017). Variations in bicarbonate may be ascribed to variable records of bicarbonate minerals in rocks and soil and their dissolution in water, microbial decomposition of organic matter and variable infiltration rate of surface run off. Percolating carbon dioxide enriched water increases the solvent action of the water due to the breakage in the pipelines (Khajuria and Dutta, 2010c).
10) Chloride
A look at the Table 2 and Fig.12 reveals that chloride varied between 11.48 mg/l (5/02/18) and 24.9 mg/l (23/04/18) and is in accordance with Reza and Singh (2009). Narrow chloride variations may be attributed to low deposits of chloride in rocks in catchment area, variable rate of sewage infiltration and variable deposits of organic matter in soil (Khajuria and Dutta, 2010b).
11) Calcium, Magnesium and Total Hardness
The study of Table 2 and Fig.13 reveals that calcium, magnesium and total hardness ranged between 24.9 mg/l (22/03/18) to 109.44mg/l (8/05/18), 4.95 mg/l (6/04/18) to 22.23 mg/l (23/05/18) and 91.2 mg/l (6/04/18) to 359.56 mg/l (8/05/18), respectively. These values are in accordance with Soni and Singh (2015). Variations in calcium, magnesium and total hardness in distribution may be attributed to entry of sewage and sediments through leakage in the pipes,
microbial decomposition of dead organic matter and deposits of biofilms inside the pipes, dead ends (Khajuria and Dutta, 2010b).
C. SITE III: Lane Tap water sample
1) Temperature
A look at Table 3 and Fig.8 reveals variation of air temperature from 17°C (20/02/18) to 32°C (23/05/18). Lowest value is observed in the month of February and highest in May of present investigation.
Water temperature ranged between 21°C (5/02/18 & 20/02/18) and 32°C (23/05/18). Thermostatic characteristics of ground water may explain narrow variation in water temperature. Increased solar radiation due to comparatively longer day length, may explain May rise in air and water temperature (Khajuria and Dutta, 2011). The difference in temperature is due to the seasonal variations and is in accordance with the findings of Parihar et al. (2012).
2) pH
pH (Table 3 and Fig.10) fluctuated between 7.41 (8/05/18) and 7.66 (22/03/18 & 23/04/18). The present range is in accordance with the findings of Sarala and Babu (2012) and Patel et al. (2017). A narrow variation in pH is due to continuous presence of free carbon dioxide and high concentration of bicarbonates (Khajuria and Dutta, 2011).
3) Electrical Conductivity
Electrical conductivity ranged from 0.266 mS/cm (5/02/18) to 0.402 mS/cm (6/04/18). April increase in electrical conductivity may be attributed to the rains (Table 3 and Fig.9). Chemostatic characteristics of ground water may explain a narrow difference of electrical conductivity and is in accordance with the findings of Sundar and Saseetharan (2008) and Khajuria and Dutta (2011).
4) Total Dissolved Solid
An observation of Table 3 and Fig.9 reveals that TDS varied between 0.2mg/l (6/04/18) – 193.7mg/l (22/03/18). Lowest value is observed in the month of February and highest in March.
5) Dissolved Oxygen
Dissolved oxygen remained fairly present throughout the study period and has shown variations from 4.45 mg/l (22/03/18) to 12.72 mg/l (7/03/18). Its highest record is in the month of March (Table 3 and Fig. 11). This type of observation has also been made earlier by Khajuria and Dutta (2010b), Usharani et al. (2010). The variation in the dissolved oxygen is due to aeration of water during pumping, variable records of temperature, free carbon dioxide, dissolved solids, microbes and decomposition of organic matter (Khajuria and Dutta, 2010b).
6) Biochemical Oxygen Demand
BOD (Table 3 and Fig. 11) varied from 1.05mg/l (20/02/18) to 5.45mg/l (7/03/18) and is in accordance with the findings of Khajuria and Dutta (2010b); Usharani et al. (2010). A good amount of dissolved oxygen in water and good filtration of water by rocks in the catchment has correlation with variable records of BOD in the ground water (Khajuria and Dutta, 2010b).
7) Free Carbon Dioxide
A look at the Table 3 and Fig.10 reveals the free carbon dioxide presence in 4 samples (7/03/18, 22/03/18, 8/05/18 & 23/05/18) and ranged between 15mg/l (23/05/18) and 17.7mg/l (22/03/18) and is in agreement with the findings of Khajuria and Dutta (2011) and Ankush (2012). Agitation of water during its pumping from ground through pipes lead to loss of free carbon dioxide and also responsible for the carbonate absence (Khajuria and Dutta, 2011).
8) Carbonate
Carbonate (Table 3 & Fig. 12) was present in 4 samples (5/02/18, 20/02/18, 6/04/18 & 23/04/18) and ranged between 4.46mg/L (5/02/18) to 12.06mg/L (20/02/18). Carbonate remains generally absent in groundwater but it is slightly present in consumer points that may be due to the entry of sewage, sediments etc in the pipes through back siphonage, cracks, dislocation, defective joints breakage etc. (Khajuria and Dutta, 2011).
9) Bicarbonate
An observation of the Table 3 and Fig. 12 reveals that bicarbonate varied between 171.72mg/l (7/03/18) to 247.62mg/l (23/04/18) and is in accordance with the findings of Joarder et al. (2008) and Khan and Rehman (2017). Its highest record is in the month of February and lowest value in the month of April. Variation in bicarbonate in ground water may be due to variable records of bicarbonate minerals in rocks and soil and their dissolution in water, carbon dioxide and organic matter in the vicinity and its decomposition (Khajuria and Dutta, 2011).
10) Chloride
Chloride (Table 3 and Fig. 12) fluctuated between 12.22mg/l (23/04/18) to 22.96mg/l (7/03/18) and is in agreement with the findings of Reza and Singh (2009) and Saleem et al. (2016). Its highest record is in the month of April and May and lowest value in the month of March. The concentration of chloride in water samples is generally due to the low deposits of chloride in rocks in catchment area, variable deposits of organic matter in the soil and sewage infiltration (Khajuria and Dutta, 2011); domestic waste, poor sanitary conditions and leaching (Mostafa et al., 2017).
11) Calcium, Magnesium and Total Hardness
An observation of the Table 3 and Fig. 13 reveals that calcium, magnesium and total hardness fluctuated between 7.74mg/l (7/03/18) and 86.85mg/l (8/05/18), 3.12mg/l (7/03/18) and 28.74mg/l (23/05/18) and 32.18mg/l (7/03/18) and 318.41mg/l (8/05/18), respectively and are in accordance with the findings of Soni and Singh (2015). Their highest record is in the month of May and lowest value in the month of March, respectively. This variation may be coincided with nature and weathering of rocks in the catchment and water depth, variable records of free carbon dioxide, variable deposits of organic matter in the soil and sewage infiltration (Khajuria and Dutta, 2011).
12) Water Quality Index (WQI)
Water Quality Index (WQI), comprising of nine water quality parameters shows the overall quality of ground water. Water Quality Index (WQI) of various physico – chemical parameters, at three stations, ranged between 36.60 (5/02/18) and 49.81 (8/05/18), 35.72 (5/02/18) and 63.31 (23/05/18), 45.34 (20/02/18) and 60.97 (22/03/18), respectively (Table 8). On the basis of Water Quality Index value, water has been categorized by Khajuria and Dutta (2010b) as:
Water Quality Index Status
0 - 25 Excellent
26 - 50 Good
51 - 75 Poor
76 - 100 Very poor
100 and above Unsuitable for drinking
All the ground water samples of site I show Water Quality Index (WQI) less than 50 and is indicative of water quality that is suitable for drinking without causing health problems. But some water samples show WQI above 50 at both consumer points which is indicative of water quality that is not suitable for drinking and can cause health problems. This is due to garbage dumping near pipelines and sewage entry (Fig. 15).
V. COEFFICIENT OF CORRELATION
Analysis of co-efficient matrix of tube well (Table 5), water samples has shown significant results with water temperature and air temperature (0.88), electric conductivity and air temperature (0.52), TDS and air temperature (0.51), DO and air temperature (-0.65), BOD and air temperature (0.54), bicarbonate and air temperature (0.79), chloride and air temperature (0.55), electric conductivity and water temperature (0.52), TDS and water temperature (0.53), BOD and water temperature (0.57), bicarbonate and water temperature (0.70), chloride and water temperature (0.59), magnesium and water temperature (0.57), DO and pH (0.65), CO2 and pH (-0.72), chloride and pH (-0.77), TDS and electric conductivity (0.99), BOD and electric conductivity (0.90), bicarbonate and electric conductivity (0.62), BOD and TDS (0.91), bicarbonate and TDS (0.61), CO2 and DO (-0.58), bicarbonate and DO (-0.71), chloride and DO (-0.63), calcium and DO (-0.68), magnesium and DO (-0.50), total hardness and DO (-0.70), bicarbonate and BOD (0.56), chloride and bicarbonate (0.88), magnesium and calcium (0.52), total hardness and calcium (0.97), total hardness and magnesium (0.70).
Analysis of co-efficient matrix of consumer point 1 (home), water samples has shown significant results with water temperature and air temperature (0.98), pH and air temperature (0.54), electric conductivity and air temperature (0.61), TDS and air temperature (-0.52), BOD and air temperature (0.68), chloride and air temperature (0.54), pH and water temperature (0.54), electric conductivity and water temperature (0.60), TDS and water temperature (-0.55), BOD and water temperature (0.62), TDS and conductivity (-0.81), bicarbonate and electrical conductivity (0.68), chloride and electric conductivity (0.86), bicarbonate and TDS (-0.67), chloride and TDS (-0.51), BOD and dissolved oxygen (0.63), CO2 and DO (0.59), calcium and DO (0.85), magnesium and DO (0.56), total hardness and DO (0.83), bicarbonate and BOD (0.57), chloride and BOD (0.54), calcium and BOD (0.79), magnesium and BOD (0.57), total hardness and BOD (0.78), bicarbonate and carbonate (-0.79), chloride and bicarbonate (0.72), magnesium and calcium (0.66), total hardness and calcium (0.97), total hardness and magnesium(0.82) (Table 6).
Analysis of co-efficient matrix of consumer point 2 (lane tap) (Table 7), water samples has shown significant results with water temperature and air temperature (0.96), conductivity and air temperature (0.73), carbonate and air temperature (-0.55), bicarbonate and air temperature (0.74), conductivity and water temperature (0.67), CO2 and water temperature (0.51), carbonate and water temperature (-0.54), bicarbonate and water temperature (0.64), calcium and pH (-0.68), magnesium and pH (-0.61), total hardness and pH (-0.71), bicarbonate and electrical conductivity (0.61), BOD and dissolved oxygen (0.82), bicarbonate and DO (0.59), chloride and DO (0.80), CO2 and BOD (0.59), carbonate and BOD (-0.62), chloride and BOD (0.70), carbonate and CO2 (-0.88), chloride and bicarbonate (-0.81), magnesium and calcium (0.67), total hardness and calcium (0.95), total hardness and magnesium (0.85).
A. Water Quality Standards
Comparison of the various physico-chemical characteristics of water with National and International Standards (WHO, 2008; BIS, 2012) reveals that all the physico-chemical parameters analyzed at three stations remain within permissible limits of drinking water standards (Table 4).
|
Parameters↓ |
||||||||
|
Air Temperature (°C) |
18 |
17 |
21 |
25 |
24.5 |
24 |
30 |
35 |
|
Water Temperature (°c) |
23 |
25 |
24 |
26 |
25.5 |
25 |
26 |
29 |
|
Ph |
6.66 |
6.89 |
7.21 |
6.99 |
6.59 |
6.92 |
6.81 |
6.77 |
|
Conductivity |
0.265 |
0.485 |
0.452 |
0.704 |
0.654 |
0.674 |
0.645 |
0.552 |
|
TDS |
0.133 |
0.25 |
0.229 |
0.354 |
0.329 |
0.339 |
0.321 |
0.281 |
|
DO (mg/L) |
4.42 |
5.38 |
5.45 |
5.4 |
4.5 |
5 |
3.63 |
4 |
|
BOD (mg/L) |
0.57 |
1.44 |
1.4 |
1.54 |
1.81 |
1.8 |
1.76 |
1.6 |
|
Carbon dioxide (mg/L) |
44.27 |
8.85 |
16 |
17 |
44.27 |
26.56 |
35.41 |
17.7 |
|
Bicarbonate (mg/L) |
315.43 |
318.92 |
269.85 |
351.52 |
364.44 |
375.52 |
409.53 |
397.29 |
|
Chloride (mg/L) |
30.83 |
31.49 |
24.6 |
31.16 |
35.26 |
33.51 |
33.86 |
35.26 |
|
Calcium (mg/L) |
111.48 |
113.36 |
52.49 |
47.29 |
65.39 |
52.74 |
197.17 |
85.92 |
|
Magnesium (mg/L) |
16.34 |
20.51 |
3.38 |
10.78 |
0.52 |
12.41 |
21.58 |
31.45 |
|
Total Hardness (mg/L) |
345.29 |
367.11 |
144.84 |
162.31 |
165.23 |
182.6 |
580.5 |
343.75 |
Table 1: Bi-monthly variations in physico-chemical parameters of Tube well water samples.
|
Parameters↓ |
||||||||
|
Air Temperature (°C) |
16 |
17 |
21 |
25 |
26 |
26 |
29 |
29 |
|
Water Temperature (°C) |
20 |
21 |
23 |
26 |
26 |
25 |
27 |
27 |
|
Ph |
7.15 |
7.49 |
7.68 |
7.5 |
7.42 |
7.54 |
7.54 |
7.65 |
|
Conductivity |
0.266 |
0.322 |
0.337 |
0.434 |
0.424 |
0.537 |
0.413 |
0.337 |
|
TDS |
132.8 |
157.8 |
168.9 |
0.212 |
0.212 |
0.269 |
0.21 |
169 |
|
DO (mg/L) |
6.73 |
7.5 |
7 |
6.27 |
3.63 |
5.45 |
10.9 |
5.45 |
|
BOD (mg/L) |
0.57 |
1.73 |
0.81 |
1.81 |
1 |
2.8 |
5.45 |
3 |
|
Carbon dioxide (mg/L) |
8.85 |
0 |
0 |
17.7 |
0 |
0 |
17.7 |
0 |
|
Carbonates (mg/L) |
0 |
12.06 |
120.65 |
0 |
7.62 |
5.35 |
0 |
8.02 |
|
Bicarbonates (mg/L) |
199.7 |
245.32 |
134.92 |
240.38 |
244.25 |
299.33 |
268.03 |
227.21 |
|
Chloride (mg/L) |
11.48 |
15.09 |
13.77 |
16.4 |
14.31 |
24.09 |
17.45 |
16.41 |
|
Calcium (mg/L) |
47.65 |
56.68 |
27.1 |
24.9 |
28.39 |
40.97 |
109.44 |
38.48 |
|
Magnesium (mg/L) |
15.52 |
19.02 |
6.51 |
8.49 |
4.95 |
8.71 |
21.05 |
22.23 |
|
Total Hardness (mg/L) |
182.73 |
219.66 |
94.42 |
97.07 |
91.2 |
138.04 |
359.56 |
187.5 |
Table 2: Bi-monthly variation in physico-chemical parameters of tap (home) water samples.
|
Samples→ |
5/02/18 |
20/02/18 |
7/03/18 |
22/03/18 |
6/04/18 |
23/04/18 |
8/05/18 |
23/05/18 |
|
Parameters↓ |
||||||||
|
Air Temperature (°C) |
20 |
17 |
23 |
26.5 |
29 |
27 |
30 |
32 |
|
Water Temperature (°C) |
21 |
21 |
26 |
26 |
28 |
28 |
30 |
32 |
|
pH |
7.47 |
7.59 |
7.59 |
7.66 |
7.51 |
7.66 |
7.41 |
7.45 |
|
Conductivity |
0.266 |
0.314 |
0.313 |
0.389 |
0.402 |
0.373 |
0.369 |
0.352 |
|
TDS |
132.8 |
164.7 |
158.8 |
193.7 |
0.2 |
186.4 |
186.5 |
174.3 |
|
DO (mg/L) |
6.73 |
6.92 |
12.72 |
4.45 |
5.45 |
7.27 |
9.09 |
5.45 |
|
BOD (mg/L) |
1.53 |
1.05 |
5.45 |
2 |
1.81 |
3 |
3.63 |
2.81 |
|
Carbon dioxide (mg/L) |
0 |
0 |
16 |
17.7 |
0 |
0 |
17 |
15 |
|
Carbonate (mg/L) |
4.46 |
12.06 |
0 |
0 |
7.62 |
6.69 |
0 |
0 |
|
Bicarbonate (mg/L) |
211.04 |
196.26 |
171.72 |
227.45 |
228.74 |
247.62 |
242.18 |
246.26 |
|
Chloride (mg/L) |
13.45 |
14.76 |
22.96 |
12.46 |
13.26 |
12.22 |
12.91 |
14.31 |
|
Calcium (mg/L) |
57.99 |
52.19 |
7.74 |
16.74 |
25.81 |
23.53 |
86.85 |
33.11 |
|
Magnesium (mg/L) |
14.98 |
21.25 |
3.12 |
11.79 |
8.34 |
9.5 |
24.73 |
28.74 |
|
Total Hardness (mg/L) |
206.27 |
217.62 |
32.18 |
90.28 |
98.71 |
97.82 |
318.41 |
200.89 |
Table 3: Bi-monthly variation in physico-chemical parameters of tap (lane) water samples.
|
Stations→ |
Station1 |
Station2 |
Station3 |
WHO(2008) |
BIS(2012) |
|||||
|
Parameters↓ |
Min. |
Max. |
Min. |
Max. |
Min. |
Max. |
Desirable Limit |
Permissible limit |
Desirable limit |
Permissible limit |
|
Air Temperature (°C) |
17 |
35 |
16 |
29 |
17 |
32 |
||||
|
Water Temperature (°C) |
23 |
29 |
20 |
27 |
21 |
32 |
||||
|
pH |
6.66 |
7.21 |
7.15 |
7.68 |
7.41 |
7.66 |
6.5-8.5 |
No relaxation |
6.5-8.5 |
No relaxation |
|
Conductivity |
0.265 |
0.704 |
0.266 |
0.537 |
0.266 |
0.402 |
1500* |
3000 |
||
|
TDS |
0.133 |
0.354 |
0.21 |
169 |
0.2 |
193.7 |
600 |
1000 |
500 |
2000 |
|
DO (mg/L) |
3.63 |
5.45 |
3.63 |
10.9 |
4.45 |
12.72 |
5-7** |
|||
|
BOD (mg/L) |
0.57 |
1.81 |
0.57 |
5.45 |
1.05 |
5.45 |
5** |
|||
|
Carbon dioxide (mg/L) |
8.85 |
44.27 |
8.85 |
17.7 |
15 |
17.7 |
||||
|
Carbonate (mg/L) |
- |
- |
5.35 |
120.65 |
4.46 |
12.06 |
||||
|
Bicarbonate (mg/L) |
269.85 |
409.53 |
134.92 |
299.33 |
171.72 |
247.62 |
300* |
600* |
300 |
600 |
|
Chloride (mg/L) |
24.6 |
35.26 |
11.48 |
24.09 |
12.22 |
22.96 |
250 |
600 |
250 |
1000 |
|
Calcium (mg/L) |
47.29 |
197.17 |
24.9 |
109.44 |
7.74 |
86.85 |
100 |
300 |
75 |
200 |
|
Magnesium (mg/L) |
0.52 |
31.45 |
4.95 |
22.23 |
3.12 |
28.74 |
30* |
150* |
30 |
100 |
|
Total hardness (mg/L) |
144.84 |
580.5 |
91.2 |
359.56 |
32.18 |
318.41 |
100 |
500 |
200 |
600 |
|
*WHO (1997), **WHO, (1993) |
||||||||||
Table 4: Comparison of water quality of Tube well, Tap (home) and Tap (lane) water with National and International standards.
|
|
Air Temperature (°C) |
Water Temperature (°c) |
pH |
EC |
TDS |
DO (mg/L) |
BOD (mg/L) |
Carbon dioxide (mg/L) |
Bicarbonate (mg/L) |
Chloride (mg/L) |
Calcium (mg/L) |
Magnesium (mg/L) |
Total Hardness (mg/L) |
|
|
Air Temperature (°C) |
1 |
|||||||||||||
|
Water Temperature (°c) |
0.88 |
1 |
||||||||||||
|
pH |
-0.16 |
-0.14 |
1 |
|||||||||||
|
Conductivity |
0.52 |
0.53 |
0.08 |
1 |
||||||||||
|
TDS |
0.52 |
0.54 |
0.08 |
1.00 |
1 |
|||||||||
|
DO (mg/L) |
-0.65 |
-0.41 |
0.65 |
-0.02 |
-0.01 |
1 |
||||||||
|
BOD (mg/L) |
0.54 |
0.57 |
0.12 |
0.91 |
0.91 |
-0.07 |
1 |
|||||||
|
Carbon dioxide (mg/L) |
0.01 |
-0.30 |
-0.72 |
-0.12 |
-0.14 |
-0.58 |
-0.20 |
1 |
||||||
|
Bicarbonate (mg/L) |
0.79 |
0.71 |
-0.49 |
0.63 |
0.62 |
-0.72 |
0.56 |
0.25 |
1 |
|||||
|
Chloride (mg/L) |
0.55 |
0.60 |
-0.77 |
0.46 |
0.46 |
-0.63 |
0.42 |
0.36 |
0.88 |
1 |
||||
|
Calcium (mg/L) |
0.17 |
0.04 |
-0.33 |
-0.14 |
-0.16 |
-0.68 |
-0.07 |
0.28 |
0.39 |
0.27 |
1 |
|||
|
Magnesium (mg/L) |
0.47 |
0.57 |
-0.20 |
-0.13 |
-0.13 |
-0.50 |
-0.09 |
-0.27 |
0.49 |
0.41 |
0.52 |
1 |
||
|
Total Hardness (mg/L) |
0.27 |
0.19 |
-0.33 |
-0.16 |
-0.17 |
-0.70 |
-0.09 |
0.16 |
0.45 |
0.33 |
0.97 |
0.71 |
1 |
|
|
|
|
|||||||||||||
|
|
Air Temperature (°C) |
Water Temperature (°C) |
pH |
EC |
TDS |
DO (mg/L) |
BOD (mg/L) |
Carbon dioxide (mg/L) |
Carbonates (mg/L) |
Bicarbonates (mg/L) |
Chloride (mg/L) |
Calcium (mg/L) |
Magnesium (mg/L) |
Total Hardness (mg/L) |
|
Air Temperature (°C) |
1 |
|||||||||||||
|
Water Temperature (°C) |
0.98 |
1 |
||||||||||||
|
pH |
0.55 |
0.55 |
1 |
|||||||||||
|
Conductivity |
0.62 |
0.61 |
0.31 |
1 |
||||||||||
|
TDS |
-0.53 |
-0.56 |
0.10 |
-0.82 |
1 |
|||||||||
|
DO (mg/L) |
-0.02 |
-0.05 |
0.09 |
-0.18 |
0.02 |
1 |
||||||||
|
BOD (mg/L) |
0.69 |
0.62 |
0.38 |
0.39 |
-0.38 |
0.63 |
1 |
|||||||
|
Carbon dioxide (mg/L) |
0.18 |
0.24 |
-0.24 |
0.08 |
-0.48 |
0.59 |
0.42 |
1 |
||||||
|
Carbonates (mg/L) |
-0.22 |
-0.22 |
0.49 |
-0.23 |
0.47 |
0.03 |
-0.36 |
-0.36 |
1 |
|||||
|
Bicarbonates (mg/L) |
0.45 |
0.42 |
-0.07 |
0.69 |
-0.68 |
0.02 |
0.58 |
0.17 |
-0.79 |
1 |
||||
|
Chloride (mg/L) |
0.55 |
0.48 |
0.42 |
0.86 |
-0.51 |
-0.02 |
0.54 |
-0.06 |
-0.25 |
0.73 |
1 |
|||
|
Calcium (mg/L) |
0.19 |
0.12 |
-0.05 |
-0.02 |
-0.18 |
0.86 |
0.79 |
0.47 |
-0.31 |
0.37 |
0.13 |
1 |
||
|
Magnesium (mg/L) |
0.06 |
0.00 |
0.01 |
-0.43 |
0.38 |
0.56 |
0.57 |
0.18 |
-0.38 |
0.19 |
-0.06 |
0.66 |
1 |
|
|
Total Hardness (mg/L) |
0.16 |
0.09 |
-0.03 |
-0.15 |
-0.02 |
0.83 |
0.79 |
0.41 |
-0.36 |
0.34 |
0.08 |
0.97 |
0.82 |
1 |
Table 5: Coefficient of correlation between physico-chemical parameters of Tube well water samples.
Table 6: Coefficient correlation between physico-chemical parameters of Tap (home) water samples.
Table 7: Coefficient correlation between physico-chemical parameters of Tap (lane) water samples
|
|
Air Temperature (°C) |
Water Temperature (°C) |
pH |
EC |
TDS |
DO (mg/L) |
BOD (mg/L) |
Carbon dioxide (mg/L) |
Carbonate (mg/L) |
Bicarbonate (mg/L) |
Chloride (mg/L) |
Calcium (mg/L) |
Magnesium (mg/L) |
Total Hardness (mg/L) |
|
Air Temperature (°C) |
1 |
|||||||||||||
|
Water Temperature (°C) |
0.96 |
1 |
|
|||||||||||
|
Ph |
-0.33 |
-0.30 |
1 |
|||||||||||
|
Conductivity |
0.73 |
0.67 |
0.18 |
1 |
||||||||||
|
TDS |
-0.07 |
0.05 |
0.22 |
-0.19 |
1 |
|||||||||
|
DO (mg/L) |
-0.22 |
-0.04 |
-0.04 |
-0.38 |
0.19 |
1 |
||||||||
|
BOD (mg/L) |
0.30 |
0.45 |
-0.02 |
0.01 |
0.27 |
0.82 |
1 |
|||||||
|
Carbon dioxide (mg/L) |
0.46 |
0.52 |
-0.14 |
0.22 |
0.49 |
0.26 |
0.59 |
1 |
||||||
|
Carbonate (mg/L) |
-0.55 |
-0.55 |
0.25 |
-0.12 |
-0.38 |
-0.24 |
-0.63 |
-0.89 |
1 |
|||||
|
Bicarbonate (mg/L) |
0.74 |
0.65 |
-0.24 |
0.61 |
0.07 |
-0.60 |
-0.24 |
0.02 |
-0.14 |
1 |
||||
|
Chloride (mg/L) |
-0.28 |
-0.11 |
0.11 |
-0.41 |
0.03 |
0.81 |
0.71 |
0.31 |
-0.24 |
-0.81 |
1 |
|||
|
Calcium (mg/L) |
-0.06 |
-0.08 |
-0.69 |
-0.23 |
0.14 |
0.00 |
-0.25 |
-0.06 |
0.08 |
0.25 |
-0.40 |
1 |
||
|
Magnesium (mg/L) |
0.23 |
0.27 |
-0.61 |
-0.06 |
0.34 |
-0.33 |
-0.30 |
0.19 |
-0.08 |
0.46 |
-0.41 |
0.67 |
1 |
|
|
Total Hardness (mg/L) |
0.05 |
0.05 |
-0.72 |
-0.18 |
0.23 |
-0.13 |
-0.29 |
0.03 |
0.03 |
0.36 |
-0.44 |
0.96 |
0.86 |
1 |
|
NARWAL |
HOME |
TAP LANE |
|
36.60 |
35.72 |
46.86 |
|
42.12 |
45.58 |
45.34 |
|
46.34 |
44.74 |
49.41 |
|
43.77 |
50.44 |
60.98 |
|
42.02 |
54.07 |
53.65 |
|
45.70 |
60.18 |
56.48 |
|
49.81 |
56.29 |
49.89 |
|
46.17 |
63.31 |
58.88 |
Table8 : WQI of various physico - chemical characteristics of Tube well, Tap (home) and Tap (lane) water samples.
WQI of various physico - chemical characteristics of Tube well, Tap (home) and Tap (lane) water samples.
Station 1 Station 2 Station 3
Figure 8: Bi-monthly variations in air and water temperature of three different sites.
Station 1 Station 2 Station 3
Figure 9: Bi-monthly variations in EC and TDS of three different sites.
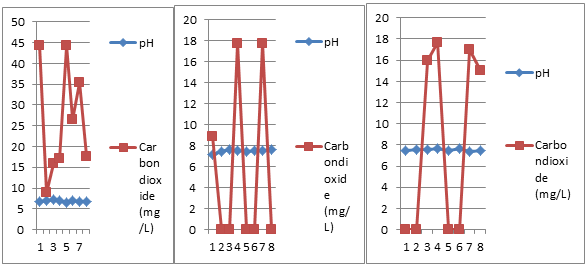
Figure 10: Bi-monthly variations in pH and CO2 of three different sites.
Station 1 Station 2 Station 3
Figure 11: Bi-monthly variations in D.O. and B.O.D. of three different sites.
Station 1 Station 2 Station 3
Figure 12: Bi-monthly variations in CO32-, HCO 3- and Cl- of three different sites.
Station 1 Station 2 Station 3
Figure 13: Bi-monthly variations in Ca, Mg and TH of three different sites.
Conclusion
A study of groundwater quality i.e. tube well and its two distribution points viz. home and lane tap, in Bathindi, was undertaken to work out the suitability for drinking purposes by studying the variations in physico-chemical and microbial parameters i.e. air temperature, water temperature, pH, electrical conductivity, carbon dioxide, dissolved oxygen, biochemical oxygen demand, total dissolved solids, bicarbonate, chloride, calcium, magnesium, total hardness and MPN. In the present study, air temperature ranged between 17°C and 35°C / 16°C and 29°C / 17°C and 32°C; water temperature 23°C and 29°C / 20°C and 27°C / 21°C and 32°C; pH 6.66 and 7.21 / 7.15 and 7.68 / 7.41 and 7.66; electrical conductivity 0.265mS/cm and 0.704mS/cm / 0.26mS/cm and 0.537mS/cm / 0.266mS/cm and 0.402mS/cm; TDS 0.133mg/L and 0.354 mg/L / 0.21 mg/L and 169 mg/L / 0.2 mg/L and 193.7 mg/L; free carbon dioxide 8.85 mg/L and 44.27 mg/L / 8.85 mg/L and 17.7 mg/L / 15 mg/L and 17.7 mg/L; bicarbonate 269.85 mg/L and 409.53 mg/L / 134.92 mg/L and 299.33 mg/L / 171.72 mg/L and 247.62 mg/L; DO 3.63 mg/L and 5.45 mg/L / 3.63 mg/L and 10.9 mg/L / 4.45 mg/L and 12.72 mg/L; BOD 0.57 mg/L and 1.81 mg/L / 0.57 mg/L and 5.45 mg/L / 1.05 mg/L and 5.45 mg/L; chloride 24.6 mg/L and 35.26 mg/L / 11.48 mg/L and 24.09 mg/L / 12.22 mg/L and 22.96 mg/L; calcium 47.29 mg/L and 197.17 mg/L / 24.9 mg/L and 109.44mg/L / 7.74 mg/L and 86.85 mg/L; magnesium 0.52 mg/L and 31.45 mg/L / 4.95 mg/L and 22.23 mg/L / 3.12 mg/L and 28.74 mg/L and total hardness 144.84 mg/L and 580.5 mg/L / 91.2 mg/L and 359.56 mg/L / 32.18 mg/L and 318.41 mg/L, at Station I / Station II / Station III, respectively. Carbonate is observed at two stations only i.e. Station II / Station III (5.35mg/L and 120.65mg/L / 4.46mg/L and 12.06mg/L). MPN, in the present study, has shown a variation from <1 to 24 at Station I; 1 to >180 at Station II and <1 to >180 at Station III.
References
[1] Ankush (2012). Groundwater quality of the two tube wells located in old and new campus, University of Jammu, Jammu. M.Sc. Dissertation (partial fulfillment towards Master’s Degree in Env. Sci.) submitted to the University of Jammu, Jammu: 1-60. [2] APHA (1998). Standards methods for the examination of water and waste water (20th edition). America. Pub. Hlth. Ass., Washington, DC.: 2005-2605. [3] Ashiyani, N.; Parekh, F. and Suryanarayana, T. M. V. (2015). Analysis of physico-chemical properties of groundwater. Int. J. Innov. Res. Sci., Eng. Tech., 4(3): 1094-1098. [4] Behailu, T. W.; Badessa, T. S. and Tewodros, B. A. (2017). Analysis of physical and chemical parameters in ground water used for drinking around Konso area, Southwestern Ethopia. J. Anal. Bioanal. Tech., 8(5): 1-7. [5] BIS: 10500 (2012). Indian standard specification for drinking water IS: 10500 -12 (Bureau of Indian standards) New Delhi, India. [6] British Ministry of Health (1957). The Bacteriological examination of water supplies. Report no. 71. Ministry of Health, London, UK. [7] Bundela, S.; Sharma, A.; Pandey, A. K.; Pandey, P. and Awasthi, A. K. (2012). Physico-chemical analysis of groundwater near municipal solid waste dumping site in Jabalpur. Int. J. Plant Animal Env. Sci., 2(1): 217-222. [8] Chaudhari, K. G. (2014).Studies of physicochemical parameters of underground water samples. Der. Chemica. Sinica., 5(1): 135-137. [9] Chavan, B. L. and Zambare, N. S. (2014). Physicochemical analysis of groundwater samples in Solapur City, Maharashtra, India. Int. J. Res. Cvl. Eng., Arch. Dsgn., 2(3): 7-12. [10] Chidinma, I.; Mathew, O.; Grace, E.; Emmanuel, N.; Chika, E.; Ifeanyichukwu, I.; Monique, A. and Emeka, I. (2016). Bacteriological and physico-chemical parameters of some selected borehole water sources in Abakaliki metropolis, Nigeria. Int. J. of Comm. Med. Pub. Hlth., 3(11): 3271-3277. [11] Das, K. C.; Roy, A. and Roy, R. (2014). Physico-chemical analysis of underground water from Silchar Municipal area of Cachar district, Assam, India. Int. J. Eng. Res. App., 4(11): 105-108. [12] Daud, M. K.; Nafees, M.; Ali, S.; Rizwan, M.; Bajwa, R. A.; Shakoor, M. B.; Arshad, M. U.; Malook, I. and Zhu, S. J. (2017). Drinking water quality status and contamination in Pakistan. Biomed. Res. Int., 1-18.
Copyright
Copyright © 2024 Nahira Shameem, Sakshi Sharma. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET65580
Publish Date : 2024-11-27
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

