Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Modern Scientific Assessment: Evaluating the Antibacterial Properties of Tulsi Leaf Nanoparticles
Authors: Reena ., Preeti Sharma, Nakuleshwar Dut Jasuja, Sunil Kumar
DOI Link: https://doi.org/10.22214/ijraset.2024.63432
Certificate: View Certificate
Abstract
In recent years, advancements in nanotechnology have enabled the development of Tulsi-based nanomedicines, enhancing the therapeutic potential of this traditional medicinal plant. This study investigates the significance of Tulsi (Ocimum sanctum) leaf nanopowder in both traditional and nanomedicine, focusing on its extensive health benefits. Known for its anti-inflammatory, antioxidant, and antimicrobial properties, Tulsi has long been valued in traditional medicine. By integrating this knowledge with nanotechnology, new opportunities for improving healthcare have emerged. Field Emission Scanning Electron Microscopy (FE-SEM) determined the average particle size to be approximately 1-10 µm, while Energy Dispersive Spectroscopy (EDS) identified the elemental composition of the powder. The antimicrobial efficacy of the Tulsi powder has been analyzed.
Introduction
I. INTRODUCTION
Tulsi powder, a form of Ayurvedic medicine, is prepared through the process of calcination, where metallic and mineral substances are heated to high temperatures. This method is thought to alter the elemental composition and physical properties of the substances, transforming them into a medicinal powder used to treat various ailments [1]. The chemical constituents of medicinal plants play a crucial role in determining their therapeutic effects and potential toxicity. Calcination can modify the elemental makeup and physical characteristics of these substances, leading to the formation of new compounds and structural changes in the original materials [2].
These alterations can influence the bioavailability, effectiveness, and safety of the final medicinal product. For instance, Ayurvedic metals like iron, copper, and zinc are believed to offer anti-inflammatory and immune-boosting benefits. However, if the bhasma (calcined preparation) is not processed correctly or contains impurities, it can result in harmful side effects. To ensure the safety and therapeutic efficacy of bhasma, careful control of the calcination process is essential, along with rigorous testing for purity, chemical composition, and potential toxicity [3]. Modern scientific tools are now used to analyze the chemical composition of these preparations. In Ayurvedic medicine, Tulsi powder is traditionally used to address various health conditions, such as respiratory issues, digestive disorders, skin problems, and infections. It is also valued as a natural remedy for stress and anxiety, believed to have a calming effect on both mind and body. Recent scientific research has validated many of these traditional applications of Tulsi powder [4].
Tulsi, also known as Holy Basil, can be consumed in various forms, such as tea or dietary supplements, and is believed to offer numerous health benefits, including its potential role in natural cancer treatment [5-7]. A literature review on Tulsi’s medicinal use highlights its active compounds, therapeutic applications, and extensive health-promoting properties. In 2023, Han Jiang and colleagues synthesized cobalt nanoparticles using Tulsi and explored their applications in wastewater treatment and biomedicine. In 2011, Mondal and colleagues investigated the immunomodulatory effects of Tulsi leaf extract in human subjects, providing valuable insights into Tulsi’s potential to regulate the immune system, which is important for immune-related disorders [8].
In 2014, Cohen authored a comprehensive review on Tulsi's medicinal properties, including its antimicrobial, anti-inflammatory, and adaptogenic effects, as well as its ability to manage stress and its therapeutic applications in various health conditions. In 2014, Cohen further reviewed Tulsi’s wide-ranging traditional uses and highlighted its stress-reducing, antimicrobial, and adaptogenic properties [9]. Given its long-standing use in Ayurvedic medicine for treating numerous diseases, further research is required to explore Tulsi’s chemical composition and physical properties with the help of modern scientific techniques. This article explores the use of Tulsi powder to utilize contemporary scientific tools to study its antimicrobial activity and further enhance its therapeutic potential.
II. MATERIALS AND METHODS
Fresh Tulsi leaves were carefully harvested and thoroughly rinsed, first under cool running water, followed by a wash with sterilized distilled water. The leaves were then spread in a single layer, ensuring adequate space between them to avoid overcrowding, as illustrated in Figure 1. They were left to air dry for eight days. Once dried, the leaves were ground into powder using a mortar. After that, filter out smooth powder and coarse powder with the help of a net. The course powder is put into the mortar and made smaller and smaller. These processes are repeated till the amount of tulsi powder is not in smooth and thin powder. Lastly, powders are stored in airtight containers for later use.
For the next phase, 50 grams of each leaf powder sample were measured and placed into separate conical flasks. Each flask was then filled with 250 milliliters of methanol, and the contents were thoroughly mixed to facilitate the extraction of active constituents into the solvent. Following a cold maceration process, the mixtures were filtered to remove any remaining solids, yielding a clear solution. The antimicrobial activity was assessed using two bacterial cultures: Bacillus subtilis and Escherichia coli.
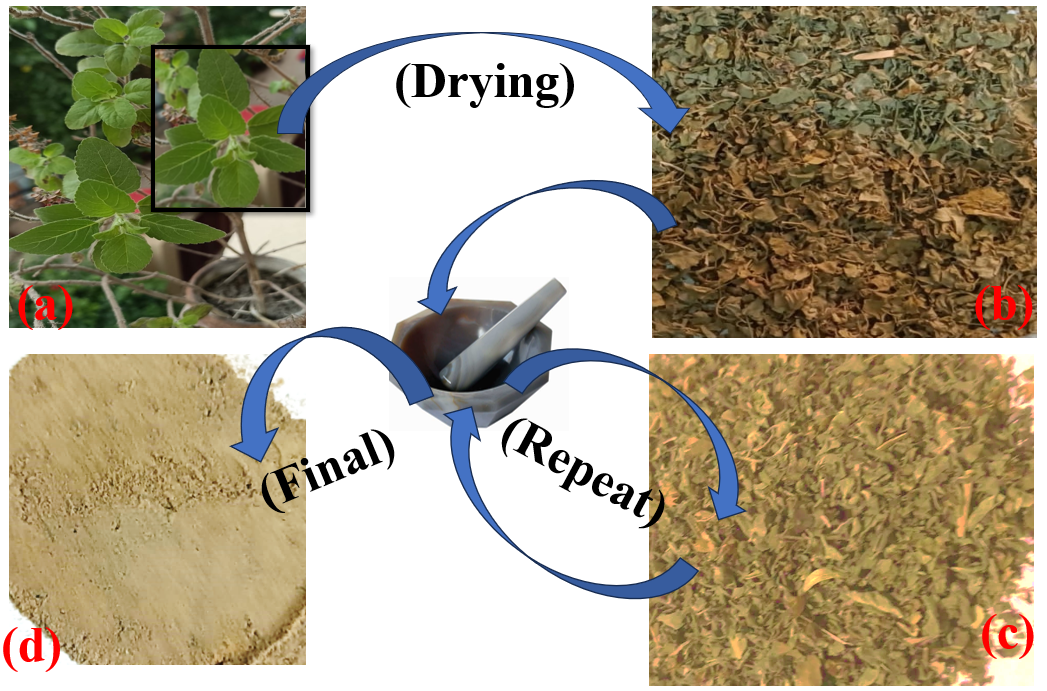 Fig. 1 (a-d). Tulsi powder stepwise preparation process.
Fig. 1 (a-d). Tulsi powder stepwise preparation process.
III. RESULTS AND DISCUSSION
Morphology analysis: The FE-SEM micrograph, along with the particle size distribution of Tulsi powder, is depicted in Figure 2 (a-d). The average particle size measured was 1-10 µm. Despite variations in individual particle sizes, the particles are evenly distributed within the Tulsi powder [10]. It is important to emphasize that although the sizes of the particles differ, their distribution remains consistent across the powder sample.

Figure 2. (a-c) FE-SEM micrograph of tulsi powder and (d) histogram corresponding to the particle size distribution of Tulsi powder.
The particle size distribution of Tulsi powder ranged from 5 µm to 10 µm, indicating that most particles fall within this size bracket, with occasional deviations outside this range. The FE-SEM micrograph provides a visual overview of this distribution, illustrating both the particle size variation and their overall dispersion within the powder [11]. An average particle size of 6-8 µm points to the presence of microscale particles, which could influence the powder's physicochemical properties and potential applications. The analysis of the FE-SEM micrograph and particle size distribution sheds light on the particle size characteristics, enhancing our understanding of the powder's physical traits and its possible uses across different fields [12].
IV. ENERGY DISPERSIVE X-RAY SPECTROSCOPY
The chemical composition of Tulsi powder used in Ayurvedic medicine is critical to ensure it does not contain harmful elements. To examine the composition of various parts of the sample, Energy Dispersive X-ray Spectroscopy (EDS) microanalysis was performed, as shown in Figure 3 [13]. The EDS has been done in a selected area from the entire sample as shown in figure 4. The EDS analysis identified elements such as carbon (C), oxygen (O), sodium (Na), magnesium (Mg), silicon (Si), sulfur (S), and chlorine (Cl) [14]. The concentrations of these elements are listed in Table I. EDS microanalysis provides a quantitative evaluation of the sample's elemental composition. Table I outlines the specific concentrations of carbon, oxygen, sodium, magnesium, silicon, sulfur, and chlorine in Tulsi powder. Ensuring that no harmful elements are present and that beneficial elements are found in appropriate amounts is essential for maintaining the quality and safety of Tulsi powder in Ayurvedic treatments [15].
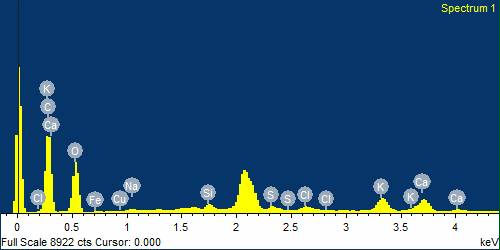
Figure 3. EDS spectrum of tulsi powder to representation of elements present
|
Table.I. Elements presents in EDS |
|
||
|
Element |
Weight% |
Atomic% |
|
|
C K |
50.28 |
59.95 |
|
|
O K |
41.15 |
36.83 |
|
|
Na K |
0.39 |
0.24 |
|
|
Si K |
0.66 |
0.34 |
|
|
S K |
0.66 |
0.30 |
|
|
Cl K |
0.56 |
0.23 |
|
|
K K |
2.42 |
0.89 |
|
|
Ca K |
2.50 |
0.89 |
|
|
Fe K |
0.78 |
0.20 |
|
|
Cu K |
0.59 |
0.13 |
|
|
Totals |
100.00 |
||
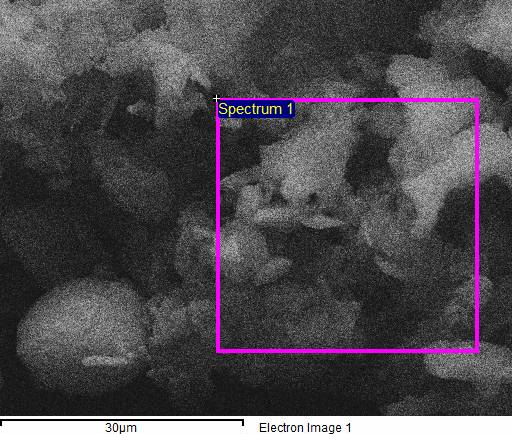
Figure 4. EDS taken from Tulsi powder.
A. FTIR Analysis
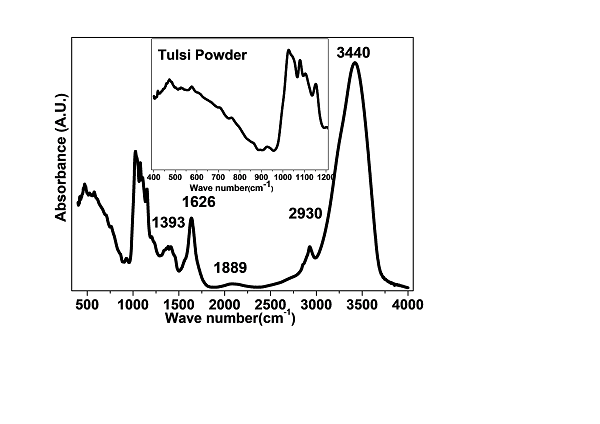
Figure 5. FTIR spectrum 400 cm-1 to 4000 cm-1 of Tulsi powder and the enlarged view of 400 cm-1 to 1200 cm-1 shown inset for clear visibility.
FTIR analysis of Tulsi powder offers crucial insights into its molecular structure, functional groups, and chemical properties, aiding in the understanding of its potential medicinal uses in Ayurveda [16-17]. To conduct the analysis, a mixture of Tulsi powder and KBr was compressed into a pellet, which was then placed in the FTIR spectrometer [18]. Infrared radiation, spanning a range of wavenumbers, was passed through the sample, resulting in the absorption or transmission of specific wavelengths. This interaction generated an infrared spectrum, which was subsequently analyzed to identify the characteristic peaks and functional groups within the Tulsi powder. The peaks were compared to reference spectra or databases to determine the sample's chemical components and structural features [19].
FTIR spectrum 400 cm-1 to 4000 cm-1 of Tulsi powder and the enlarged view of 400 cm-1 to 1200 cm-1 shown inset for clear visibility as shown in figure 5. This analysis of Tulsi powder revealed essential information about its molecular composition and the presence of various functional groups [20]. The infrared spectrum was used to identify key peaks, which in turn allowed the characterization of organic compounds in the sample. Peaks observed in the spectrum can be attributed to specific functional groups based on their characteristic frequencies. For instance, peaks around 3300-3500 cm?¹ suggest the presence of hydroxyl groups, while those in the 1650-1750 cm?¹ range indicate carbonyl groups (C=O) [21]. Typically, FTIR analysis identified peaks corresponding to functional groups such as hydroxyl (OH), carbonyl (C=O), aromatic compounds, aliphatic compounds, and other chemical bonds [22].
The absorption peaks corresponding to specific chemical bonds, such as C-H stretching, C-O stretching, and C-C stretching, along with their vibrational modes, are in figure 5. Through the analysis of the FTIR spectrum, valuable information about the chemical composition of Tulsi powder can be obtained, facilitating the identification and characterization of key organic compounds [23]. This knowledge contributes to a deeper understanding of the medicinal properties and potential applications of Tulsi in Ayurvedic medicine.
C. Antimicrobial Activity
Tulsi powder extract was prepared by mixing a specified weight or volume of the powder with an appropriate solvent, such as water, ethanol, or methanol, depending on the extraction method [24]. The concentration of the extract can vary based on the target test concentration. To assess its antimicrobial properties, different microorganisms are often selected.
In this study, Bacillus subtilis and Escherichia coli. were used as model organisms to evaluate the antimicrobial activity of the Tulsi extract [25]. These bacteria were grown in suitable culture media until they reached the desired growth phase. The microbial density was then adjusted to achieve the required inoculum size, which was determined either by measuring optical density or through viable count methods, such as colony-forming units per milliliter (CFU/mL), using agar plates [26].
The antimicrobial susceptibility of the selected microorganisms was assessed using techniques like agar well diffusion or disc diffusion. In these methods, the test microorganisms were inoculated onto agar plates, wells were created in the agar, and the Tulsi extract was introduced into these wells [27]. The plates were then incubated under optimal conditions for the respective microorganisms to observe the antimicrobial effect of the extract.
As outlined in the materials and methods section, nutrient agar plates were prepared and inoculated with the specified microorganisms using the spread plate technique. The preparation of nutrient agar followed standard protocols, and the bacterial inoculum was evenly spread across the agar surface using a sterile spreader. Wells of 6 mm in diameter were created on the agar using a sterile punch, serving as the sites for introducing the Tulsi powder extracts. As previously described, the extracts were prepared at various concentrations (200 mg/mL, 400 mg/mL, 600 mg/mL, 800 mg/mL).
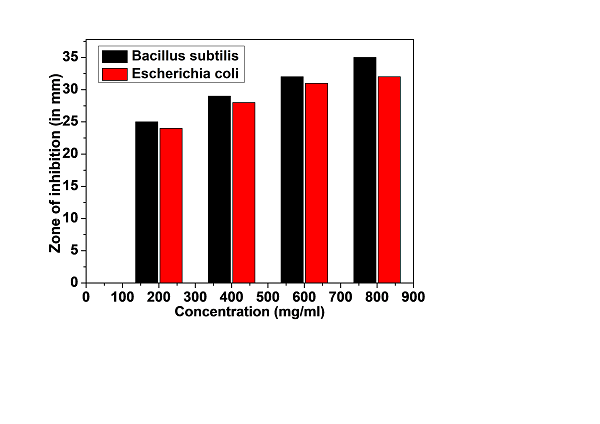
Fig. 6. These zones (mm) indicate the degree of inhibition or growth inhibition caused by the Tulsi powder extract.
Using a micropipette, 50 µL of each Tulsi extract concentration was transferred into the respective wells on the agar plates [28]. The plates were incubated at 37°C for 24 hours to allow the antimicrobial compounds in the extracts to diffuse into the surrounding agar. Following incubation, the plates were examined for zones of inhibition (ZOIs), which appeared as clear, circular regions around the wells where bacterial growth was suppressed due to the antimicrobial action of the Tulsi extracts [29].
The diameter of the ZOIs was measured with a Vernier scale or ruler marked in millimeters. The widest point of each ZOI was recorded, providing a quantitative measure of the extract's antimicrobial effectiveness [30-31]. A larger ZOI diameter indicated stronger antimicrobial activity against the tested microorganisms [32]. Statistical analysis was performed to assess the significance of the results, as illustrated in Figure 6.
V. DISCUSSION
FESEM micrograph of the Tulsi powder revealed a close-packed microstructure with average particle 1-10 µm. The chemical composition estimated by EDX of the Tulsi powder such as Carbon (C), oxygen (O), sodium (Na), magnesium (Mg), silicon (Si), sulphur (S), chlorine (Cl) and no trace of any harmful elements. The combination of these elements gives rise to various functional groups. The minimum inhibitory concentration (MIC) for both microorganisms was 800 mg/ml in the antimicrobial test. Tulsi powder extract was shown to have a more powerful effect on Bacillus subtilis and Escherichia coli.
Conclusion
Modern scientific research tools have provided valuable insights into Tulsi powder\'s physical and medicinal properties. The FESEM micrograph demonstrated a uniform particle distribution across the surface of the Tulsi powder, with an average particle size of 1-10 µm. EDS analysis identified and quantified the elements present in the powder, including carbon (C), oxygen (O), sodium (Na), magnesium (Mg), silicon (Si), sulfur (S), and chlorine (Cl), while no harmful elements were detected. FTIR analysis helped identify key compounds in Tulsi, such as phenols, flavonoids, terpenes, and other organic molecules, contributing to its medicinal properties. These experimental findings provide Ayurvedic practitioners with enhanced knowledge that can assist in treating various diseases. Antimicrobial studies of Tulsi nanoparticles have shown their effectiveness against bacteria, suggesting their potential in controlling bacterial infections. A. Acknowledgements The authors extend sincere gratitude and acknowledge IIT Delhi for providing access to their experimental facility available at the Central Research Facility (C. R. F.). B. Financial Support and sponsorship Nil. C. Conflicts of Interest There are no conflicts of interest.
References
[1] Cohen, M. (2014). Tulsi - Ocimum sanctum: A herb for all reasons. Journal of Ayurveda and Integrative Medicine, 5(4), 251-259. https://doi.org/10.4103/0975-9476.146554 [2] Srivastava, A. K. (2021). Tulsi (Ocimum sanctum): A Potent Adaptogen. Clinical Research Notes, 2(2), 01–05. https://doi.org/10.31579/2690-8816/037 [3] Hasan, M. R., Alotaibi, B. S., Althafar, Z. M., Mujamammi, A. H., & Jameela, J. (2023). An update on the therapeutic anticancer potential of Ocimum sanctum L.: “Elixir of Life.” Molecules, 28(3), 1193. https://doi.org/10.3390/molecules28031193 [4] Prakash, P., & Gupta, N. (2005). Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: A short review. Indian Journal of Physiology and Pharmacology, 49(2), 125-131. PMID: 16353802. [5] Hasan, M. R., Alotaibi, B. S., Althafar, Z. M., Mujamammi, A. H., & Jameela, J. (2023). An update on the therapeutic anticancer potential of Ocimum sanctum L.: “Elixir of Life.” Molecules, 28(3), 1193. https://doi.org/10.3390/molecules28031193 [6] Jiang, H., Sathiyavimal, S., Cai, L., Devanesan, S., Sayed, S. R. M., Jhanani, G. K., & Lin, J. (2023). Tulsi (Ocimum sanctum) mediated Co nanoparticles with their anti-inflammatory, anti-cancer, and methyl orange dye adsorption properties. Environmental Research, 236(Pt 2), 116749. https://doi.org/10.1016/j.envres.2023.116749 [7] Mondal, S., et al. (2011). Double-blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. Journal of Ethnopharmacology, 136(3), 452-456. [8] Baliga, M. S., et al. (2013). Ocimum sanctum L. (Holy Basil or Tulsi) and its phytochemicals in the prevention and treatment of cancer. Nutrition and Cancer, 65(sup1), 26-35. [9] Lahon, K., & Das, S. (2011). Hepatoprotective activity of Ocimum sanctum alcoholic leaf extract against paracetamol-induced liver damage in Albino rats. Pharmacognosy Research, 3, 13-18. [10] Singh, N., Hoette, Y., Miller, R., et al. (2010). Ocimum sanctum Linn. (Holy Basil): An overview. Journal of Chemical and Pharmaceutical Research, 2(1), 62-72. [11] Goyal, C. K., Joshi, N., & Sharma, K. C. (2022). Physico-chemical standardization and nutritional assessment of Devdarvadyarishta. Journal of Ayurveda, 16, 126-133. [12] Alzohairy, M. A. (2016). Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evidence-Based Complementary and Alternative Medicine, 2016, 7382506. [13] Al-Jadidi, H. S., & Hossain, M. A. (2015). Studies on total phenolics, total flavonoids, and antimicrobial activity from the leaves crude extracts of neem traditionally used for the treatment of cough and nausea. Beni Suef University Journal of Basic and Applied Sciences, 4, 2314-8535. [14] Suryanarayana, C. (1998). X-ray Diffraction: A Practical Approach. CRC Press. [15] Cullity, B. D., & Stock, S. R. (2001). Elements of X-ray Diffraction (3rd ed.). Prentice Hall. [16] Bragg, W. L., & Bragg, W. L. (1952). The Analysis of Crystal Structures. G. Bell and Sons Ltd. [17] Pecharsky, V. K., & Zavalij, P. Y. (2005). Fundamentals of Powder Diffraction and Structural Characterization of Materials. Springer. [18] Goldstein, J. I., Newbury, D. E., Joy, D. C., et al. (2017). Scanning Electron Microscopy and X-ray Microanalysis. Springer. [19] Reimer, L., & Kohl, H. (2008). Transmission Electron Microscopy: Physics of Image Formation. Springer. [20] Joy, D. C., & Romig, A. D. Jr. (1998). Introduction to Scanning Electron Microscopy. Oxford University Press. [21] Goldstein, J. I., Newbury, D. E., Echlin, P., et al. (2018). Scanning Electron Microscopy and X-ray Microanalysis (4th ed.). Springer. [22] Singh, S., Gautam, A., & Batra, A. (2014). FTIR spectroscopy of Ocimum sanctum (Tulsi) leaves. International Journal of Current Microbiology and Applied Sciences, 3(6), 384-394. [23] Ahmed, S., Sheraz, M. A., Tariq, M., et al. (2016). FTIR spectroscopy for identification of bioactive compounds in Ocimum sanctum (Tulsi) leaves extract. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 153, 143-148. [24] Jayaprakasha, P., Palanichamy, G., Sahoo, M. R., et al. (2023). Determination of quality standards and evaluation of In vitro antibacterial activity of Kanchanara guggulu: An Ayurvedic formulation. Journal of Ayurveda, 17, 3-9. [25] Suresh, D., Prakash, M. R., & Patel, S. B. (2011). Fourier Transform Infrared (FTIR) Spectroscopy of Tulsi (Ocimum sanctum) for the Detection of Phytocomponents. Journal of Chemical, Biological, and Physical Sciences, 1(1), 67-73. [26] Thakur, R., Naik, R., & Avangapur, S. (2020). COVID-19: Clinical aspects and role of single herbal drugs – A review from classical texts of Ayurveda. Journal of Ayurveda, 14, 103-111. [27] Kaimal, V. S., Chandran, A. S., Vineeth, P. K., & Pillai, U. (2022). In vitro study to compare the antibacterial activity of Kaphaketu Rasa and its combination with Rasa Sindura in selective respiratory pathogens. Journal of Ayurveda, 16, 87-91. [28] Pimploy, N., & Pornsawai, P. (2022). RSC Adv., 12, 26435-26454. [29] Lubis, M. F., Syahputra, H., & Astyka, R. (2022). Antibacterial Activity of Ethanolic Extract of Ocimum basilicum L. Leaves in Inhibiting the Growth of Escherichia coli and Pseudomonas aeruginosa. Nusantara Scientific Medical Research Journal, 1(1), 01-08. https://doi.org/10.58549/nsmrj.v1i1.15 [30] Panchal, P., & Parvez, N. (2019). Phytochemical analysis of medicinal herb (Ocimum sanctum). International Journal of Nanomaterials, Nanotechnology and Nanomedicine, 5(2), 008-011. https://doi.org/10.17352/2455-3492.000029 [31] Yamani, H. A., Pang, E. C., Mantri, N., & Deighton, M. A. (2016). Antimicrobial Activity of Tulsi (Ocimum tenuiflorum) Essential Oil and Its Major Constituents against Three Species of Bacteria. Frontiers in Microbiology, 7, 681.Iqbal, E., Salim, K. A., & Lim, L. B., Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. Journal of King Saud University - Science, 2015; 27(3), 224–232. [32] Khaleel Z I, Aminu F, Mu’azu L, Ali Muhammad, Antibacterial Activity and Phytochemical Screening of Acacia Nilotic a Leaf Extracts Against Clinical Isolates of Some Bacteria. J Biop Theo Stud. 2021; 1(1): 01-06.
Copyright
Copyright © 2025 Reena ., Preeti Sharma, Nakuleshwar Dut Jasuja, Sunil Kumar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63432
Publish Date : 2024-06-24
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

