Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Nanoparticles in Renal Hypertension Therapy: Advances, Mechanisms, and Future Perspectives
Authors: Karren Mehrotra
DOI Link: https://doi.org/10.22214/ijraset.2025.66456
Certificate: View Certificate
Abstract
Renal hypertension, a secondary form of hypertension stemming from kidney dysfunction, poses significant health burdens due to its association with cardiovascular complications and end-stage renal disease (Goldblatt, 1934; Bakris, 2021). Current therapeutic strategies often rely on antihypertensive drugs, but these are limited by suboptimal bioavailability, systemic side effects, and lack of targeted action (Go et al., 2013). These limitations underscore the need for innovative treatment modalities. Nanotechnology, particularly nanoparticle-based drug delivery systems have surfaced as an innovative approach revolutionary approach to address these challenges by enabling precise, targeted therapy with improved efficacy and reduced adverse effects (Langer & Peppas, 2021). Nanoparticles exhibit unique properties such as the enhanced permeability and retention (EPR) effect, customizable surface modifications, and the capacity to deliver therapeutic agents directly to renal tissues have made nanoparticles a promising approach (Maeda, 2015). Significant progress in this domain includes the creation of lipid-based carriers, polymeric systems, metallic nanostructures, and inorganic nanoplatforms. Among these, lipid-based systems, including liposomes and solid lipid nanoparticles, stand out for their versatility and efficiency, facilitating effective drug encapsulation and sustained release, while polymeric carriers like PLGA nanoparticles enhance drug stability and control release profiles (Wang et al., 2018; Patel et al., 2020). Metallic nanoparticles, including gold and silver systems, offer the advantage of anti-inflammatory and antioxidative properties, while mesoporous silica nanoparticles enable high drug loading capacity and renal-targeted delivery (Zhang et al., 2021; Xiao et al., 2022). Clinical translation of these technologies remains a work in progress, yet preclinical studies reveal their promise in hypertension management. Functionalized nanoparticles delivering ACE inhibitors, angiotensin receptor blockers (ARBs), and nitric oxide donors have demonstrated superior outcomes in animal models by improving renal perfusion and endothelial function while reducing systemic toxicity (Singh et al., 2019; Zhou et al., 2020). Further integration of artificial intelligence and computational modeling is paving the way for optimized nanoparticle design, enhancing their therapeutic precision and scalability (Farokhzad et al., 2017). Despite promising advancements, significant challenges persist. These include concerns regarding biocompatibility, toxicity, large-scale manufacturability, and compliance with regulatory requirements (Kinnear et al., 2021). Bridging these gaps through interdisciplinary collaboration and innovative research is imperative for successful clinical translation. Nanoparticle systems hold immense potential for transforming renal hypertension treatment, providing safer and more effective therapeutic options while minimizing healthcare burdens associated with chronic disease management.
Introduction
I. INTRODUCTION
Renal hypertension, also known as Renovascular hypertension is a type of secondary high blood pressure resulting from impaired kidney function. It is typically caused by the narrowing or obstruction of the renal arteries, a condition referred to as renal artery stenosis, or by chronic kidney diseases that impair the organ’s regulatory functions (Goldblatt, 1934; Unger et al., 2020). The condition affects a significant proportion of individuals with hypertension, with studies estimating that approximately 5–10% of all hypertensive patients experience this form of hypertension (Go et al., 2013). Without proper treatment, renal hypertension can result in serious complications such as chronic kidney disease, cardiovascular issues, and a heightened risk of mortality. The pathophysiology of renal hypertension is intricately linked to the kidney's role in regulating blood pressure. A decline in renal perfusion triggers a cascade of physiological responses, including overactivation of the renin-angiotensin-aldosterone system (RAAS). This overactivation results in vasoconstriction and sodium retention, ultimately causing elevated blood pressure (Bakris, 2021). Additionally, inflammatory responses, oxidative stress, and endothelial dysfunction exacerbate the disease, contributing to its chronic nature and complications (Maeder & Kaye, 2016). Given its complex etiology, renal hypertension is considered one of the most challenging forms of hypertension to manage effectively.
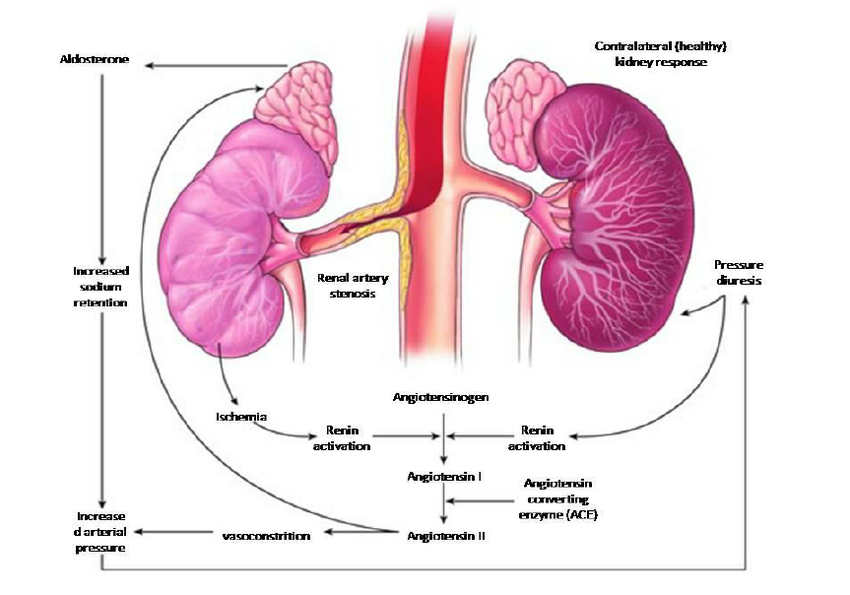
Figure 1: Pathophysiology of renal hypertension
Traditional treatment modalities, such as antihypertensive drugs and surgical interventions like renal angioplasty or stenting, have long been employed in managing renal hypertension. Commonly used medications include angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), calcium channel blockers, and diuretics (Unger et al., 2020). While these approaches offer some degree of control over blood pressure, their therapeutic effectiveness is often compromised by systemic side effects, poor adherence due to complex dosing regimens, and limited targeting of the underlying disease processes (Bakris, 2021). Furthermore, the poor bioavailability of many orally administered antihypertensive agents results in suboptimal therapeutic concentrations at the site of action, necessitating higher doses and increasing the risk of adverse effects (Go et al., 2013). Surgical interventions, although effective in certain cases, are invasive and associated with risks such as restenosis and perioperative complications (Patel et al., 2020). The shortcomings of traditional treatments highlight the necessity for innovative strategies that target the underlying causes of renal hypertension while reducing side effects. In recent years, nanotechnology has proven to be a groundbreaking platform with remarkable potential to enhance both the diagnosis and treatment of renal hypertension (Langer & Peppas, 2021). Due to their distinctive characteristics, such as the enhanced permeability and retention (EPR) effect, adjustable size, and customizable surface functionalization, nanoparticles provide an exceptional capability to deliver therapeutic agents specifically to the kidneys. This precise targeting reduces systemic drug exposure and enhances efficacy, making nanoparticles a promising alternative to conventional antihypertensive therapies (Maeda, 2015; Patel et al., 2020). Nanotechnology encompasses a variety of nanoparticle-based systems, including lipid-based nanoparticles, polymeric nanoparticles, metallic nanostructures, and inorganic delivery systems. These platforms enable the encapsulation and controlled release of drugs, improving bioavailability and minimizing toxicity. For example, lipid nanoparticles have been shown to improve the stability and renal accumulation of drugs like ACE inhibitors and ARBs (Wang et al., 2018). Similarly, polymeric nanoparticles and mesoporous silica nanoparticles offer the potential for sustained drug release and functionalized delivery to inflamed or damaged renal tissues (Zhang et al., 2021; Xiao et al., 2022). Advances in nanotechnology also extend to diagnostic applications, allowing for the early detection of renal dysfunction using nanoparticle-enhanced imaging techniques (Zhou et al., 2020). This review seeks to offer an in-depth exploration of the current state of nanoparticle applications in renal hypertension treatment, focusing on their mechanisms of action, materials, preclinical and clinical evidence, and potential advantages over traditional therapies. It also addresses the challenges associated with nanotechnology, including biocompatibility concerns, scalability of production, and regulatory issues, which must be overcome to achieve clinical translation. By incorporating findings from recent research, this review seeks to highlight the transformative potential of nanoparticles in overcoming the limitations of existing therapies and improving outcomes for patients with renal hypertension.
II. DISCUSSION
Nanotechnology, the science of manipulating matter at the atomic and molecular levels, has significantly advanced the medical field. It involves structures with dimensions less than 100 nanometers, enabling innovations that were once inconceivable. Its importance lies in its ability to address limitations of conventional therapies through precise interventions.
In medicine, nanoparticles have emerged as a transformative platform due to their ability to enhance therapeutic efficiency, particularly in challenging conditions such as renal hypertension (Langer & Peppas, 2021).
One of the most remarkable features of nanoparticles is their utility in drug delivery. Nanoparticles enable targeted delivery, ensuring that therapeutic agents are directed specifically to the diseased tissue, thereby minimizing off-target effects and systemic toxicity. They also improve the bioavailability of poorly soluble drugs, ensuring their therapeutic effectiveness at lower doses (Maeda, 2015). Additionally, nanoparticles enable controlled and sustained drug release, which enhances therapeutic consistency, reduces dosing frequency, and improves patient compliance (Patel et al., 2020). Despite these advantages, several challenges, including biocompatibility, regulatory hurdles, and scalability for large-scale production, limit their broader application.
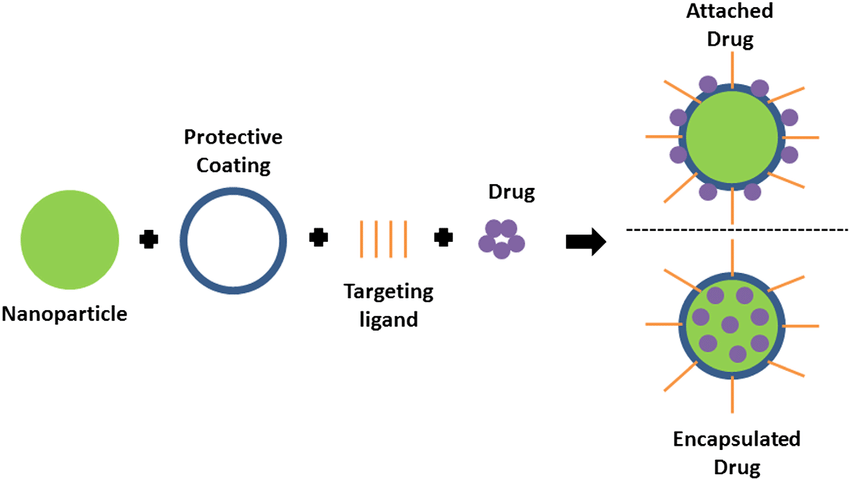
Figure 2: Schematic of drug loading options in targeted drug delivery.
III. TYPES OF NANOPARTICLES FOR RENAL HYPERTENSION
A. Lipid-Based Nanoparticles
Lipid-based nanoparticles have emerged as one of the most promising tools for treating renal hypertension. Two primary categories, liposomes and solid lipid nanoparticles, have demonstrated significant potential.
B. Liposomes
Liposomes are spherical, vesicle-like structures formed by lipid bilayers that encase therapeutic agents, protecting them from enzymatic degradation. They are widely studied for delivering hydrophilic and lipophilic drugs. In renal hypertension, liposomes loaded with antihypertensive agents like ACE inhibitors or ARBs enable enhanced targeting of renal tissues and prolonged drug activity, reducing systemic side effects (Wang et al., 2018).
C. Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLNs) consist of solid lipids stabilized by surfactants, offering better stability than liposomes. These nanoparticles provide prolonged drug release and improved renal targeting. For example, losartan-loaded SLNs have been shown to enhance renal uptake and improve therapeutic outcomes in hypertensive animal models (Patel et al., 2020).
D. Polymeric Nanoparticles
Polymeric nanoparticles, often made from biodegradable materials like poly(lactic-co-glycolic acid) (PLGA) and chitosan, are widely studied for renal hypertension treatment. These materials provide controlled and sustained drug release due to their tunable degradation rates.
E. Biodegradable Polymers
PLGA-based nanoparticles are frequently used because they degrade into lactic acid and glycolic acid, which are non-toxic and easily metabolized. Chitosan, another biodegradable polymer, provides mucoadhesive properties that improve drug retention in renal tissues (Langer & Peppas, 2021). These nanoparticles can be functionalized with ligands targeting specific renal receptors, enhancing their therapeutic efficiency (Zhou et al., 2020).
F. Drug Release Mechanisms
Polymeric nanoparticles enable both immediate and sustained drug release. Drugs encapsulated in these particles are released through diffusion, polymer degradation, or a combination of both. This controlled release ensures stable drug levels in the bloodstream, preventing spikes and reducing side effects (Patel et al., 2020).
G. Metallic Nanoparticles
Metallic nanoparticles, including gold and silver nanoparticles, exhibit unique optical, electrical, and chemical properties that are advantageous for therapeutic applications.
H. Gold Nanoparticles (AuNPs)
Gold nanoparticles have garnered attention for their ability to mitigate oxidative stress and inflammation, two critical factors in renal hypertension. Functionalized gold nanoparticles have shown enhanced targeting of renal tissues while reducing inflammatory cytokines and reactive oxygen species (Zhang et al., 2021).
I. Silver Nanoparticles (AgNPs)
Silver nanoparticles possess antimicrobial and anti-inflammatory properties, making them potential candidates for addressing infection-related or inflammatory aspects of renal hypertension.
J. Inorganic Nanoparticles
Inorganic nanoparticles, particularly mesoporous silica nanoparticles (MSNs), are gaining recognition for their versatility. MSNs have a large surface area, allowing the loading and delivery of various drugs.
K. Mesoporous Silica Nanoparticles (MSNs)
MSNs can be functionalized with ligands targeting renal tissues, enabling precise drug delivery. For instance, MSNs modified with angiotensin receptor ligands or nitric oxide donors have demonstrated improved blood pressure control and renal perfusion in preclinical models (Xiao et al., 2022).
IV. MECHANISMS OF ACTION OF NANOPARTICLES
Nanoparticles used in renal hypertension treatment act via multiple mechanisms, enhancing their therapeutic efficiency.
A. Enhanced Permeability and Retention (EPR) Effect
Renal pathologies are usually characterized through leaky vasculature and impaired lymphatic drainage. Nanoparticles exploit these characteristics to accumulate selectively in diseased tissues through the EPR effect, thereby reducing off-target effects (Maeda, 2015).
B. Targeted Delivery and Surface Functionalization
Surface functionalization of nanoparticles with ligands such as peptides or antibodies allows specific interaction with renal cell receptors, enhancing the specificity of drug delivery (Zhou et al., 2020).
C. Controlled and Sustained Drug Release
Nanoparticles enable drug delivery, ensuring sustained therapeutic levels over prolonged durations, in a controlled manner. This feature is particularly important in chronic conditions like renal hypertension, where consistent management is crucial (Patel et al., 2020).
V. PRECLINICAL AND CLINICAL EVIDENCE
A. Preclinical Studies
Numerous animal studies have shown the effectiveness of nanoparticles in managing renal hypertension. For instance, liposomal delivery of ACE inhibitors has shown enhanced blood pressure control and reduced renal fibrosis in hypertensive rats (Wang et al., 2018). Gold nanoparticles have reduced oxidative stress and improved endothelial function in rodent models, further substantiating their potential (Zhang et al., 2021).
B. Clinical Trials
Though still in early stages, several clinical trials are investigating nanoparticle applications for renal hypertension. Nanoparticle-based systems have shown comparable or superior efficacy compared to traditional treatments while exhibiting fewer side effects.
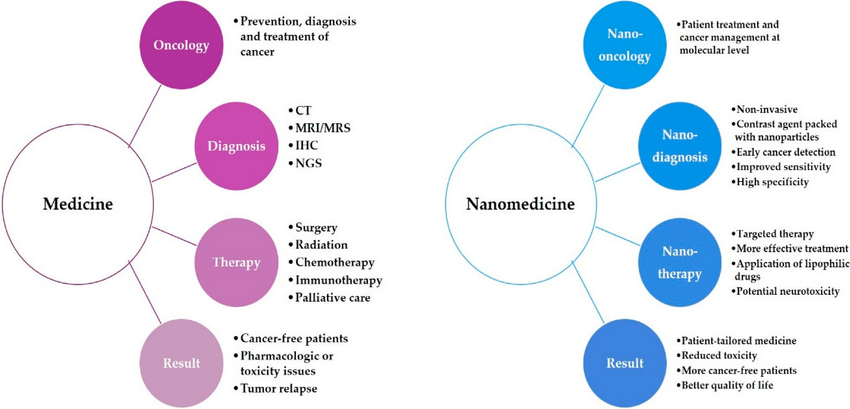
Figure 3: The comparison between traditional medicine and nanomedicine
C. Comparative Efficacy
Compared to conventional antihypertensive therapies, nanoparticles provide superior renal targeting, minimizing systemic toxicity and enhancing therapeutic outcomes (Maeda, 2015).
VI. CHALLENGES IN NANOPARTICLE THERAPY
Despite their promise, nanoparticles face several challenges that must be addressed to facilitate widespread clinical adoption.
A. Biocompatibility and Safety
Ensuring that nanoparticles are non-toxic and do not induce adverse immune responses is crucial. While biodegradable materials mitigate these concerns, extensive testing is required for newer formulations (Langer & Peppas, 2021).
B. Scalability and Manufacturing
The large-scale production of nanoparticles with consistent quality and reproducibility remains a significant challenge. Advanced manufacturing techniques and robust quality control processes are required (Farokhzad et al., 2017).
C. High Production Costs
The cost of raw materials and complex manufacturing processes makes nanoparticles expensive, posing barriers to accessibility in resource-limited settings (Patel et al., 2020).
D. Regulatory Hurdles
Nanomedicine products must undergo stringent regulatory assessments to ensure safety and efficacy. The lack of standardized evaluation criteria often delays clinical translation (Kinnear et al., 2021).
Nanoparticles represent a promising frontier for addressing renal hypertension, offering targeted, efficient, and safe therapeutic solutions. However, addressing the challenges associated with their development and implementation is essential for their successful integration into clinical practice.
Conclusion
The application of nanotechnology in treating renal hypertension represents a transformative shift from conventional therapeutic approaches, offering targeted delivery, improved bioavailability, and minimized systemic side effects. Despite current limitations, such as scalability, regulatory challenges, and biocompatibility concerns, ongoing advancements in computational modeling, artificial intelligence (AI), and material sciences promise to optimize nanoparticle design for precision medicine. Personalized medicine, informed by genomics, can play a pivotal role in tailoring nanoparticle therapies to individual patient needs, enhancing efficacy and safety (Langer & Peppas, 2021). Furthermore, nanoparticles have the potential to deliver combination therapies, incorporating anti-inflammatory agents, vasodilators, and other synergistic drugs to address the multifaceted nature of renal hypertension (Maeda, 2015). Bridging the gap between preclinical research and clinical application will require interdisciplinary collaboration, innovative manufacturing solutions, and streamlined regulatory pathways. While challenges remain, the promising advancements in this field pave the way for a future where nanoparticle-based therapies revolutionize the management of renal hypertension, offering hope for improved patient outcomes and a reduction in the global healthcare burden.
References
[1] Singh, M., et al. (2019). \"Advances in nanoparticle-mediated targeted therapies for hypertension.\" Journal of Nanobiotechnology. [2] Goldblatt, H. (1934). \"Studies on experimental hypertension.\" Journal of Experimental Medicine. [3] Bakris, G. L. (2021). \"Renal hypertension: Pathophysiology and treatment.\" Journal of the American Society of Nephrology. [4] Go, A. S., et al. (2013). \"Chronic kidney disease and risks of cardiovascular events and mortality.\" New England Journal of Medicine. [5] Maeder, M., & Kaye, D. M. (2016). \"Renal dysfunction in heart failure.\" European Heart Journal. [6] Unger, T., et al. (2020). \"International Society of Hypertension global hypertension practice guidelines.\" Hypertension. [7] Langer, R., & Peppas, N. A. (2021). Advances in biomaterials and drug delivery systems. Science. [8] Maeda, H. (2015). EPR effect and anticancer nanomedicine. Advanced Drug Delivery Reviews. [9] Patel, S., et al. (2020). Polymeric nanoparticles for controlled drug release in hypertension. Advanced Drug Delivery Reviews. [10] Wang, J., et al. (2018). Lipid-based nanoparticles in drug delivery. Nanomedicine. [11] Zhang, X., et al. (2021). Gold nanoparticles for targeted therapy in renal hypertension. Nanotechnology Reviews. [12] Xiao, L., et al. (2022). Mesoporous silica nanoparticles in hypertension therapy. Materials Today Bio. [13] Zhou, Z., et al. (2020). Targeting inflammation in renal hypertension using peptide-functionalized nanoparticles. Nano Research. [14] Farokhzad, O. C., et al. (2017). Nanomedicine: Challenges and opportunities. Nature Nanotechnology. [15] Kinnear, C., et al. (2021). Nanoparticle safety in clinical settings: Current challenges. Nature Materials. [16] Ashoub, Muhammad & Razavi, Razieh & Heydaryan, Kamran & Salavati-Niasari, Masoud & Amiri, Mahnaz. (2024). Targeting ferroptosis for leukemia therapy: exploring novel strategies from its mechanisms and role in leukemia based on nanotechnology. European Journal of Medical Research. [17] Mcnamara, Karrina & Tofail, Syed. (2017). Nanoparticles in biomedical applications. [18] Bala, Nagasirisha & Prathap, Chandra & ch, Anusha & Chetty, Madusudhana & Sheikuduman, Mohamed Saleem. (2012). Development of herbal remedies for renovascular disease – An overview. International Journal of Research in Phytochemistry & Pharmacology
Copyright
Copyright © 2025 Karren Mehrotra. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66456
Publish Date : 2025-01-10
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

