Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Use of Nanotechnology in Cosmetics and Cosmeceuticals
Authors: Pallavi Shivaji Ghule, Om Sanjay Nagare, Baleshwari Chandrakant Jagtap, Savita Dattu Daunde
DOI Link: https://doi.org/10.22214/ijraset.2024.64488
Certificate: View Certificate
Abstract
Nanotechnology has revolutionized the cosmetic and cosmeceutical industries by enhancing product efficacy and enabling innovative formulations. This review paper delves into the various applications of nanotechnology in skincare and cosmetic products, highlighting its ability to improve the delivery of active ingredients, provide enhanced protection, and increase the bioavailability of beneficial compounds. Nanomaterials such as liposomes, nanoemulsions, nanocapsules, and solid lipid nanoparticles are now extensively utilized for their superior skin penetration capabilities, ensuring targeted delivery and prolonged effects. The paper explores the potential benefits and safety concerns associated with these nano-based cosmetic products. It also provides a comprehensive analysis of recent advancements, regulatory considerations, and future trends in the field, emphasizing the need for further research to fully understand the long-term implications of nanotechnology in cosmetics and cosmeceuticals. This review aims to provide a critical overview for researchers and industry professionals interested in the evolving landscape of nanotechnology in beauty and skincare. Findings Our research highlights the transformative impact of Artificial Intelligence (AI) and Machine Learning (ML) on the drug discovery and development process, particularly in enhancing efficiency and precision from initial screening to clinical trials. The integration of AI/ML has shown significant advancements in early-stage drug discovery, where data-driven algorithms enable rapid identification of potential drug candidates, reducing reliance on traditional, labor-intensive methods. In the drug design and optimization phase, AI-driven predictive models have streamlined the process, minimizing the need for extensive physical testing by accurately simulating drug interactions and predicting possible side effects. Additionally, AI and ML are revolutionizing clinical trials by optimizing trial design, improving patient recruitment and retention, and enhancing real-time data monitoring, leading to faster and more reliable trial outcomes. These technologies also support personalized medicine approaches and have proven essential in reducing both the time and cost associated with bringing new therapies to market. Overall, our findings underscore the critical role of AI and ML in reshaping the pharmaceutical landscape, making drug development faster, more cost-effective, and ultimately, more successful in delivering effective treatments to patients.
Introduction
I. INTRODUCTION
Nanotechnology, a multidisciplinary field involving the manipulation of materials at the nanoscale, has gained significant traction across various industries, including medicine, electronics, and energy. In recent years, the cosmetic and cosmeceutical sectors have embraced nanotechnology for its potential to enhance product performance and functionality. The unique properties of nanomaterials—such as their small size, large surface area-to-volume ratio, and ability to penetrate biological barriers—make them ideal for improving the delivery and efficacy of active ingredients in skincare and beauty products. The use of nanotechnology in cosmetics and cosmeceuticals has led to the development of advanced formulations designed to overcome traditional limitations, such as poor skin absorption and instability of active compounds.1,2,3 By encapsulating active ingredients in nanocarriers like liposomes, nanoemulsions, and solid lipid nanoparticles, manufacturers can achieve targeted delivery, controlled release, and increased bioavailability of substances like vitamins, antioxidants, and anti-aging agents. In addition to enhancing efficacy, nanotechnology offers other benefits, such as improved texture, stability, and UV protection in cosmetic formulations.4,5,6 These innovations have prompted rapid growth in the nano-based beauty market, with consumers seeking more effective and long-lasting solutions for skin care, anti-aging, and sun protection. However, the incorporation of nanotechnology in cosmetics and cosmeceuticals also raises important questions regarding safety and regulatory oversight. The long-term effects of nanomaterials on human health and the environment remain under investigation, and regulatory agencies are working to establish guidelines for the safe use of these materials in consumer products.7,8,9 This review aims to provide a comprehensive overview of the current state of nanotechnology in cosmetics and cosmeceuticals, discussing its benefits, challenges, and future directions. By exploring the advancements and ongoing research in this area, this paper seeks to shed light on how nanotechnology is reshaping the cosmetic industry and its potential impact on consumer health and safety
II. EXPLORING THE BENEFITS AND RISKS OF SUNSCREENS AND COSMETICS"
Titanium dioxide and zinc oxide sunscreens are widely used to shield the skin from harmful solar radiation, including various UV rays.10,11,12 These sunscreens typically contain zinc oxide (ZnO) and titanium dioxide (TiO2) as inorganic UV filters, which block the penetration of damaging sunlight. ZnO is particularly effective in blocking UVA rays, while TiO2 is more suited for the UVB range. Combining these particles in proper ratios ensures broad-spectrum UV protection. TiO2, especially at the nanoscale, is one of the most used inorganic nanoparticles in sunscreens, providing a higher sun protection factor (SPF) and enhanced effectiveness due to its transparency, which is linked to its large surface-area-to-volume ratio at the nano level. TiO2 and ZnO nanoparticles, starting at nanoscale sizes, offer improved skin protection and appearance, but their inhalation has been identified as harmful. For this reason, dermal application in sunscreens is considered safer, with no evidence suggesting their penetration into the epidermis or posing significant toxicity risks. The International Agency for Research on Cancer (IARC) classifies TiO2 as a possible carcinogen, while ZnO is deemed safe for use by the USFDA as a UV filter in cosmetics. As a safer alternative, naturally occurring nanoparticles like those secreted by the roots of English ivy offer UV protection with enhanced visual transparency, making them a favorable substitute to reduce health and environmental impact. Gold and silver nanoparticles are known for their antibacterial and antifungal properties and are commonly incorporated into cosmetic formulations such as antiperspirants, anti-aging creams, and face masks.14,15,16,17 Historically, gold has been used for skin health, with ancient Egyptians employing it to maintain complexion. In modern skincare, gold is used in products like creams and treatments, often in the form of colloidal gold or nanogold, which varies in size and color depending on the nanoparticle's characteristics.18Gold nanoparticles have diverse shapes and are known for their stability, biocompatibility, and skin-rejuvenating properties. They are especially effective in healing skin damage, reducing inflammation, and offering anti-aging benefits. Silver nanoparticles, on the other hand, are utilized for their ability to inhibit microbial growth and have demonstrated stability in cosmetic formulations. However, the safety of silver nanoparticles, particularly in European and US markets, remains under evaluation. Silica nanoparticles, which have hydrophilic surfaces and are cost-effective to manufacture, are increasingly used in the cosmetic sector. These nanoparticles improve the texture and durability of cosmetic products, including lipsticks and other skin formulations. Silica nanoparticles are versatile in delivering both hydrophilic and lipophilic ingredients, although concerns about their safety, particularly regarding toxicity, require further long-term research. Carbon black is a key ingredient in many cosmetic products, particularly as a colorant in eye and skin applications. In its nanostructured form, it has been approved for use in the EU under controlled conditions to avoid inhalation risks, as studies have indicated its potential to cause cytotoxicity and inflammation when inhaled. Nano-hydroxyapatite, used primarily in oral care products, is an effective agent for treating dental sensitivity and enamel remineralization. It is considered a promising alternative to fluoride and has been approved by the USFDA for safe use in oral formulations like toothpaste and mouthwash. Nanotechnology has also introduced innovative solutions in sunscreen formulations, with agents like tris-biphenyl triazine, a photostable UV filter, being commonly used in nano form for broad-spectrum protection. Other novel nanoparticles, such as carbon fullerene (buckyballs), are employed for their antioxidant properties in skin-rejuvenating formulations, aiding in reducing the effects of UV-induced skin damage.
III. RISKS
Despite the numerous advantages nanotechnology offers in cosmetics, concerns regarding the health risks of nanoparticles remain. Due to their small size, nanoparticles can penetrate biological membranes and tissues, potentially causing toxic effects. Their increased chemical reactivity at the nanoscale can lead to oxidative stress and damage to cells, proteins, and DNA. Various human systems, including respiratory, cardiovascular, and immune systems, can be affected by exposure to nanoparticles, which can enter the body through inhalation, ingestion, or dermal application. While nanotechnology has unlocked many possibilities for more effective cosmetic formulations, understanding and mitigating the potential health risks remain critical areas of research. Environmental Risks of Nanoparticles Nanotechnology, while beneficial in many environmental applications such as reducing waste and emissions, presents significant environmental risks due to the unique properties of nanoparticles. Their ability to penetrate small spaces and cause biochemical interference in biological systems raises concerns. 19,20These particles can bioaccumulate, generate reactive oxygen species (ROS), and induce oxidative stress, which may lead to cellular damage. Environmental exposure to nanoparticles, particularly through water, air, and soil during the manufacturing process, poses a risk due to their mobility and stability. Nanoparticles such as TiO2, commonly used in products like sunscreens, are of particular concern. When these nanoparticles are released into water, they can be toxic to marine life. For example, TiO2 in sunscreen has been shown to negatively impact algae and aquatic organisms due to the breakdown of its protective coating in water. Additionally, carbon-based nanomaterials are known to accumulate in organs such as lungs and kidneys, causing cytotoxic effects in both humans and animals. These materials can disrupt microbial metabolism and affect the biogeochemical cycle of nutrients, posing further ecological risks. Studies have also highlighted the potential for nanoparticles to harm plants and enter the food chain. Metal-based nanoparticles like Ag and ZnO are absorbed by plants and algae, hindering growth and seed germination, which may lead to biomagnification across ecosystems. Research suggests that fullerene nanoparticles can be harmful to aquatic species like largemouth bass, and they exhibit bactericidal properties, which disrupt ecological balances. Efforts to mitigate the environmental impact of nanoparticles include green technology initiatives aimed at producing eco-friendly nanoparticles with reduced raw materials, energy consumption, and waste production. Green chemicals and energy-efficient procedures are employed to reduce the ecological footprint of nanoparticle production. Additionally, biosynthesis of new nanomaterials, polymeric coatings to prevent leaching, and recycling methods are suggested as potential solutions to the environmental risks posed by nanotechnology. Regulatory Guidelines for Cosmetics and Cosmeceuticals With the growing cosmetic market, which reached a value of USD 532.43 billion in 2017 and is projected to reach USD 805.61 billion by 2023, regulatory oversight is crucial to ensure consumer safety. 23The increasing prevalence of personalized cosmetic products presents challenges in fulfilling legal obligations, but proper regulatory frameworks can help ensure compliance. Further research is needed to evaluate the long-term environmental and health effects of nanomaterials, establish accurate risk assessments, and develop sustainable practices for nanotechnology in the cosmetic industry
IV. OBSERVATIONS OF NANOPARTICLES WITH ITS PROPERTIES
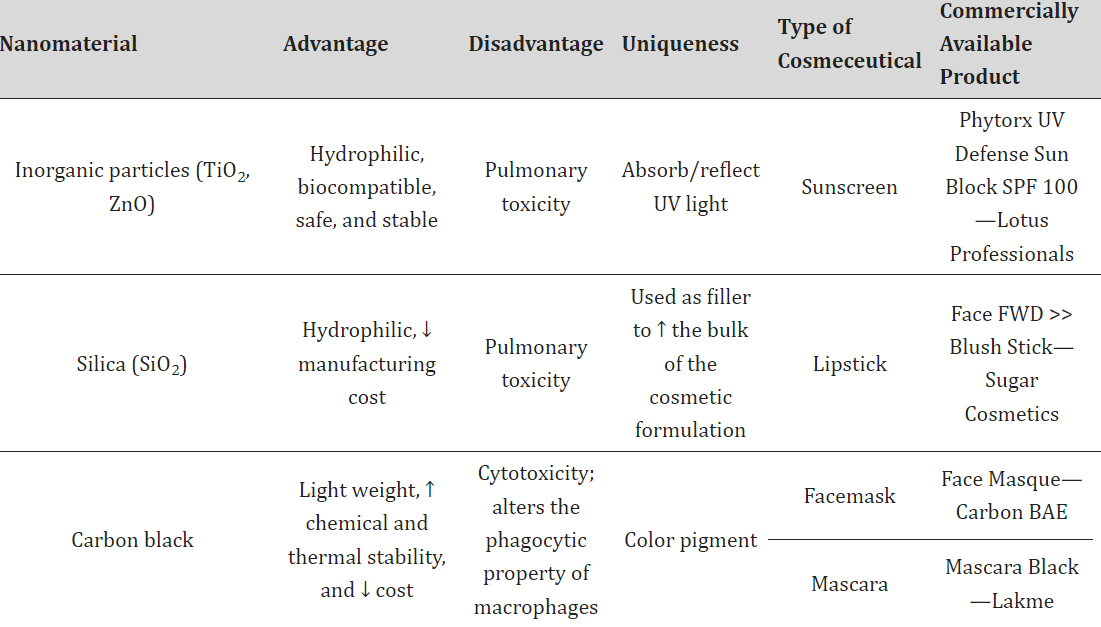
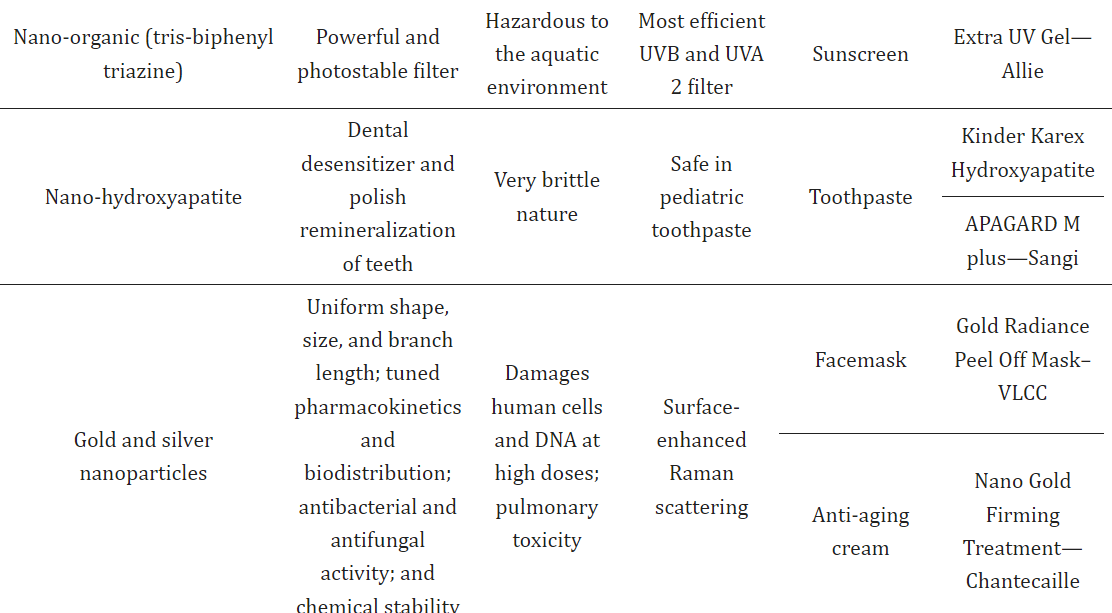
Conclusion
Nanotechnology is now recognized as a revolutionary field with significant potential, particularly in cosmetics, cosmeceuticals, dermatology, and biomedical applications. Recent innovations and advanced drug delivery systems have contributed to the growing popularity and market share of cosmetics and cosmeceuticals. Today, these products have become essential in daily routines, and the integration of nanotechnology has increased their global acceptance. However, the associated toxicity, due to the high penetrability of nanoparticles, is a major concern that is often overlooked, leading to potential health risks. Currently, novel nanocarriers such as liposomes, ethosomes, cubosomes, nano-lipid carriers (NLC), solid lipid nanoparticles (SLNs), nanoemulsions, and niosomes are being used to enhance the effectiveness of various cosmetic and cosmeceutical formulations. These nanosystems deliver active ingredients across the skin through different mechanisms, providing benefits such as sun protection, moisturization, and wrinkle reduction. Despite their impressive market growth, the safety and toxicity of these nanomaterials in humans remain a topic of intense debate, highlighting the need for more comprehensive research. To ensure consumer safety, cosmetic regulations should include detailed reference lists and clearly identify ingredients that may pose unintended environmental risks for both consumers and professionals. Long-term toxicity studies, including research on the carcinogenicity of nanocosmetics and cosmeceuticals, should be conducted before these products are commercialized. Nanocosmeceuticals must be manufactured with a focus on enhancing consumer health, and clinical trials, similar to those required for pharmaceuticals, should be carried out to ensure their safety for human use. Moreover, strict regulations should govern the manufacturing, storage, import, and marketing of cosmeceuticals and their associated nanoparticles. Global collaboration among researchers and regulatory agencies is essential for developing standardized rules and guidelines for the use of nanosystems in cosmetics. Non-governmental organizations and government bodies should also work together to create educational materials for consumers, such as written guides and videos, or host seminars to promote the responsible use of nanocosmetics and cosmeceuticals. Finally, there is a critical need for international harmonization of regulations to create a more robust framework that ensures the safety, efficacy, and marketing of these products. This would benefit both the cosmetics industry and consumers by minimizing potential hazards. Additionally, raising consumer awareness about the risks and benefits of these products would empower individuals to make more informed choices.24,25
References
[1] Raj S., Jose S., Sumod U.S., Sabitha M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied Sci. 2012;4:186–193. doi: 10.4103/0975-7406.99016. [PMC free article] [PubMed] [CrossRef] [Google Scholar] [2] Kaul S., Gulati N., Verma D., Mukherjee S., Nagaich U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018;2018:3420204. doi: 10.1155/2018/3420204. [PMC free article] [PubMed] [CrossRef] [Google Scholar] [3] Ajazzuddin M., Jeswani G., Jha A. Nanocosmetics: Past, Present and Future Trends. Recent Patents Nanomed. 2015;5:3–11. doi: 10.2174/1877912305666150417232826. [CrossRef] [Google Scholar] [4] Size L.B.M., Report S., Size L.B.M., Application B., Region B., Forecasts S. Get Free, Instant, and Unlimited Access to a PDF Sample Report & Personalized Online Dashboard. [(accessed on 15 January 2022)];2021 :2021–2028. Available online: https://main.mohfw.gov.in/sites/default/files/Annual%20Report%202020-21%20English.pdf [5] Schneider G., Gohla S., Schreiber J., Kaden W., Schönrock U., Schmidt-lewerkühne H., Kuschel A., Petsitis X., Pape W., Ippen H., et al. Connect with Wiley. The Wiley Network. 2021. [(accessed on 15 January 2022)]. pp. 2–3. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a24_219 [6] Cosmetics—Overview. [(accessed on 15 January 2022)]; Available online: https://www.fda.gov/industry/regulated-products/cosmetics-overview [7] Tripathy S., Dureja H. Cosmetics: Regulatory Scenario in USA, EU and India. J. Pharm. Technol. Res. Manag. 2015;3:127–139. doi: 10.15415/jptrm.2015.32010. [CrossRef] [Google Scholar] [8] Kumar N., Kanchan T., Unnikrishnan B., Thapar R., Mithra P., Kulkarni V., Holla R., Bhagwan D., Radhakrishnan Y. Characterization of Rubia cordifolia L. root extract and its evaluation of cardioprotective effect in Wistar rat model. Indian J. Pharmacol. 2018;49:344–347. doi: 10.4103/ijp.IJP. [CrossRef] [Google Scholar] [9] Haryanti R. Krim Pemutih Wajah dan Keamanannya. Majalah Farmasetika. 2017;2:5–9. doi: 10.24198/farmasetika.v2i3.15888. [CrossRef] [Google Scholar] [10] Search Worldwide, Life-Sciences Literature. 2021. [(accessed on 15 January 2022)]. pp. 1–2. Available online: https://www.cosmeticsandtoiletries.com/regulations/regional/article/21834383/comparatively-speaking-cosmetic-vs-cosmeceutical-vs-drug [11] Fatima M., Monawwar S., Mohapatra S., Alex T.S., Ahmed A., Taleuzzaman M., Ali A., Ansari M.J., Mirza M.A., Iqbal Z. In silico drug screening based development of novel formulations for onychomycosis management. Gels. 2021;7:221. doi: 10.3390/gels7040221. [PMC free article] [PubMed] [CrossRef] [Google Scholar] [12] Santos A.C., Morais F., Simões A., Pereira I., Sequeira J.A.D., Pereira-Silva M., Veiga F., Ribeiro A. Nanotechnology for the development of new cosmetic formulations. Expert Opin. Drug Deliv. 2019;16:313–330. doi: 10.1080/17425247.2019.1585426. [PubMed] [CrossRef] [Google Scholar] [13] Dhawan S., Sharma P., Nanda S. Nanocosmetics. Elsevier; Amsterdam, The Netherlands: 2020. Cosmetic nanoformulations and their intended use. [Google Scholar] [14] Hoag S.W., Hussain A.S. The impact of formulation on bioavailability: Summary of workshop discussion. J. Nutr. 2001;131:1389S–1391S. doi: 10.1093/jn/131.4.1389S. [PubMed] [CrossRef] [Google Scholar] [15] Souto E.B., Fernandes A.R., Martins-Gomes C., Coutinho T.E., Durazzo A., Lucarini M., Souto S.B., Silva A.M., Santini A. Nanomaterials for skin delivery of cosmeceuticals and pharmaceuticals. Appl. Sci. 2020;10:1594. doi: 10.3390/app10051594. [CrossRef] [Google Scholar] [16] Fytianos G., Rahdar A., Kyzas G.Z. Nanomaterials in cosmetics: Recent updates. Nanomaterials. 2020;10:979. doi: 10.3390/nano10050979. [PMC free article] [PubMed] [CrossRef] [Google Scholar] [17] Pandey P., Dahiya M. A Brief Review on Inorganic Nanoparticles. J. Crit. Rev. 2016;3:18–26. [Google Scholar] [18] Saxena P., Chandra A. Black carbon. Pollut. Eng. 2011;43:1–11. doi: 10.1002/9783527809080.cataz02167. [CrossRef] [Google Scholar] [19] Mohapatra S., Mirza M.A., Hilles A.R., Zakir F., Gomes A.C., Ansari M.J., Iqbal Z., Mahmood S. Biomedical application, patent repository, clinical trial and regulatory updates on hydrogel: An extensive review. Gels. 2021;7:207. doi: 10.3390/gels7040207. [PMC free article] [PubMed] [CrossRef] [Google Scholar] [20] Lee H.S., Byun S.H., Cho S.W., Yang B.E. Past, present, and future of regeneration therapy in oral and periodontal tissue: A review. Appl. Sci. 2019;9:1046. doi: 10.3390/app9061046. [CrossRef] [Google Scholar] [21] Nguyen T.A., Rajendran S. Current Commercial Nanocosmetic Products. Elsevier; Amsterdam, The Netherlands: 2020. [Google Scholar] [22] Alaqad K., Saleh T.A. Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs. J. Environ. Anal. Toxicol. 2016;6:4. doi: 10.4172/2161-0525.1000384. [CrossRef] [Google Scholar] [23] Lamberti M., Zappavigna S., Sannolo N., Porto S., Caraglia M. Advantages and risks of nanotechnologies in cancer patients and occupationally exposed workers. Expert Opin. Drug Deliv. 2014;11:1087–1101. doi: 10.1517/17425247.2014.913568. [PubMed] [CrossRef] [Google Scholar] [24] Metrics P. Fullerene is effective against wrinkles. J. Am. Acad. Dermatol. 2010;62:AB22. doi: 10.1016/j.jaad.2009.11.127. [CrossRef] [Google Scholar] [25] Bakry R., Vallant R.M., Najam-ul-Haq M., Rainer M., Szabo Z., Huck C.W., Bonn G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007;2:639–649. [PMC free article] [PubMed] [Google Scholar]
Copyright
Copyright © 2024 Pallavi Shivaji Ghule, Om Sanjay Nagare, Baleshwari Chandrakant Jagtap, Savita Dattu Daunde. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64488
Publish Date : 2024-10-07
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

