Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
NGS and Drug Development Pipeline for Alzheimers Disease
Authors: Uma Kumari, Sudhanva Devaprasad Dixit
DOI Link: https://doi.org/10.22214/ijraset.2023.55081
Certificate: View Certificate
Abstract
Alzheimer\\\'s disease is a form of dementia characterized by the gradual and irreversible loss of cognitive abilities, primarily affecting memory, thinking, and behaviour.Globally, there are more than 50 million people that suffer from Alzheimer\\\'s.Alzheimer\\\'s disease, a complex and devastating neurodegenerative disorder, represents a profound challenge to modern neuroscience and medicine. Defined by the accumulation of abnormal protein aggregates, such as beta-amyloid plaques and tau tangles, within the brain, it inexorably disrupts essential neuronal connections and communication. This relentless deterioration leads to a gradual deterioration of cognitive functions, memory loss, and ultimately, a profound loss of independence for afflicted individuals. Unravelling the intricate molecular and cellular mechanisms underlying Alzheimer\\\'s remains an urgent scientific endeavour, offering hope for novel treatments and preventive strategies to confront this formidable adversary of the human brain. In this study, the variability of the human ?-secretase, 5FN5protein sequence was compared and evaluated with the reference sequence using MMDB, BLAST, and COBALT. RasMol was utilized to identify the domain and function of the human ? secretase for the analysis of structural properties. Molecular docking was performed to study the interaction of 5FN5 molecule and the associated ligandusing PyMOL. In the computer-aided drug designing approach, molecular docking was performed for 5FN5 protein, and CB-Dock was used to find optimized conformations between 5FN5 protein and E2012 drug, resulting in the minimum energy that bound to a particular protein. E2012 was found to be the accurate drug ligand for the binding site of gamma secretase with docking score of –9, acting as a modulator for 5FN5 protein.
Introduction
I. INTRODUCTION
Alzheimer's disease (AD) is a complex and chronic neurodegenerative disease, it in the main impacts the brain, leading to a gradual decline in cognitive abilities, memory loss, and changes in behaviour. AD is the most common cause of dementia, accounting for approximately 60-80% of all cases around the world [1].Alzheimer’s has affected over 40 million people worldwide [2]. AD is the fourth greatest cause of death in the United States, trailing after heart disease, cancer, and stroke, and it is a serious public health issue impacting the entire world [3].Alzheimer’s disease involves two major proteins: β-amyloid protein and tau protein.These proteins become abnormal and toxic to the brain, by formingbeta-amyloid plaques and neurofibrillary tangles.Beta-amyloid plaques are present in between the neurons. These plaques are the clumps, formed by the buildupof insoluble fragments of a protein called amyloid-beta [4].On the other hand, the abnormal Tau protein accumulates, and forms twisted fibers, known asneurofibrillarytangles inside the neurons. As the level of abnormalβ-amyloid reaches the critical point, there will be rapid spread of Tau throughout the Brain [5].Theseplaques and tangles disrupt the normal functioning of brain cells and lead to their degeneration and eventual death.
β-amyloid (Aβ) forms plaques outside the cells and tau forms tangles inside the neurons. Both the proteins damage nerve cells causing them to die. But β-amyloid protein and tau protein may not be the only factors involved in Alzheimer’s disease [6].There are other factors to be considered.Sometimes, vascular system may fail to deliver sufficient blood and nutrients to the brain, the brain may lack glucose needed to power its activity [7]. Chronic inflammation sets in as microglial cells fail to clear away debris, furthermore astrocytes react to distressed microglia. Eventually, nerve cells lose their ability to communicate. As all the neurons die, it causes the brain to shrink. In Alzheimer’s, Hippocampus is the first part of the brain to be affected. The hippocampus is a region of the brain that is in responsible for memory and learning. Initially, there will be memory loss, impaired decision-making, and language problems [8].
As more and more neurons die, and more areas of the brain are affected, the symptoms of Alzheimer's become worse and more numerous. This is sometimes known as a gradual progression of symptoms. The brain of a person with Alzheimer's disease also has lower levels of a certain neurotransmitter which allows messages to pass between the Nerve cells.
Lower levels of neurotransmitters may cause the remaining cells to communicate with each other less effectively and it may lead to greater problems with memory and thinking. Consequently, a person with Alzheimer's disease loses the ability to remember, think, make decisions, and function independently [9].The symptoms of Alzheimer's disease typically start with mild memory loss and confusion, which gradually worsen over time. As the disease progresses, individuals may experience difficulties with language, problem-solving, and everyday tasks. Behavioural changes, mood swings, and loss of independence are also common as the disease advances [10].
The main drug treatment for Alzheimer's disease aim to increase the levels of neurotransmitters in the remaining neurons helping to preserve brain function for a while. As Alzheimer's disease progresses, it becomes very much difficult to remember things.Research indicates that abnormalities in the processing and clearance of beta-amyloid protein play a central role in the development and progression of Alzheimer's disease [11].Normally, the brain has mechanisms to remove excess beta-amyloid, but in Alzheimer's disease, this clearance process becomes impaired, leading to the accumulation of beta-amyloid plaques. These plaques trigger inflammation and oxidative stress, further damaging neurons and promoting neurodegeneration.While there is currently no cure for Alzheimer's disease, various treatments and interventions can help manage symptoms and improve quality of life for affected individuals. These may include medications to temporarily alleviate cognitive symptoms, behavioural therapies, and support from caregivers. Ongoing research aims to develop disease-modifying therapies that target the underlying mechanisms of Alzheimer's disease, with the goal of slowing down or halting its progression [12].
5FN5 is a protein structure identifier for Human g-secretase. g secretase is a protease enzyme located within the cell membrane. It is responsible for cleaving various substrates. Gamma secretase plays a crucial role in cellular processes by cleaving various substrates, including Notch and amyloid precursor protein (APP). Abnormal cuts of Notches are usually implicated in cancer, while abnormalities in cleaving β-amyloid precursor protein will lead to Alzheimer’s disease. There are gamma secretase inhibitors for Alzheimer’s such as L-685,458, DAPT [13], E2012, and begacestatwhich may work efficiently and could be one of the effectivetreatments for Alzheimer’s disease [14].5FN5 is a membrane protein that comes under the group of hydrolases. Understanding the structural characteristics and dynamics of gamma secretase in the apo-state ensemble, including class 3, is important for gaining insights into its functional mechanisms, regulation, and potential therapeutic targets. It helps to investigate the behaviour and interactions of the enzyme in its natural state, leading to a better understanding of its biological functions and potential implications in diseases like Alzheimer's disease and cancer. This human gamma secretase, 5fn5 is present and is expressed in humans. 5FN5 is composed of 4 macromolecules: Nicastrin, Presenilin-1, Gamma-secretase subunit APH-1A, and Gamma-secretase subunit PEN-2.This study is performed to identify the 5FN5(gamma secretase) inhibitors for Alzheimer’s disease [15].
II. MATERIALS AND METHODS
The study utilized the National Center for Biotechnology Information (NCBI) to obtain the amino acid sequences in FASTA format. NCBI serves as a valuable bioinformatics resource in the field of biology and biotechnology, offering access to a vast array of biological information and tools. In this research study, National Center for Biotechnology Information (NCBI) was used as a resource to retrieve the sequences[16].Later on, BLAST (Basic Local Alignment Search Tool) program was used to determine sequence similarity. BLAST only does "local" alignments.BLAST is designed to analyze and compare biological sequences. It is a powerful tool for identifying regions of similarity between different sequences, such as DNA, RNA, or protein sequences. BLAST helps in determining the degree of similarity between a query sequence and subject sequence [17].
After that, the Protein Data Bank (PDB) was used to access the repository of 5FN5, which contains information about the experimentally determined 5FN5 protein structures, the study of protein function, interactions, and structure-based drug design. Ligands were found in the literature and were downloaded in SDF format using PubChem. It is the world's largest publicly accessible chemical data archive. The PDB collects and curates data from various sources, including X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and electron microscopy. Each structure in the PDB is assigned a unique alphanumeric identifier called the PDB ID, which is used to refer to a specific entry[18].Moreover,MMDB(Molecular Modeling Database) was used to retrieve the comprehensive information on experimentally determined 3D structure of 5FN5 molecule,including its atomic coordinates, protein-ligand interactions, secondary structure annotations, and related metadata, facilitating detailed analysis and investigations on 5FN5, Cryo-EM structure of gamma secretase in class 3 of the apo- state ensemble[19]. Each chain of 5FN5 molecule was run in BLAST and Multiple sequence alignment was performed using COBALT (Constraint-Based Alignment Tool). COBALT incorporates pairwise constraints from various sources to generate accurate and reliable alignments, making it particularly useful for studying the evolution and relationships between proteins[20].RasMolwas used to visualize the 3D structure of 5FN5.
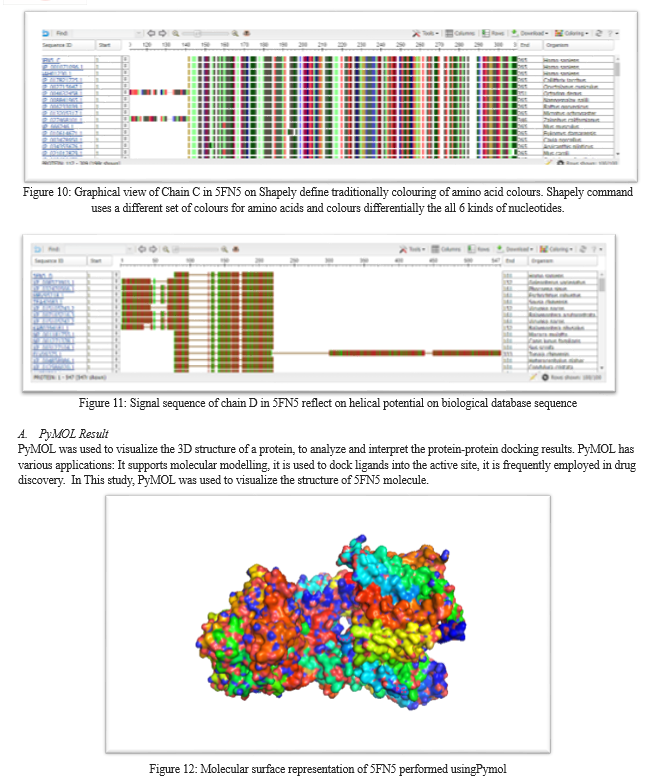
RasMol usually helps in visualizing the three-dimensional structures of molecules, such as proteins, nucleic acids, and small molecules, which are typically obtained from experimental techniques like X-ray crystallography or nuclear magnetic resonance (NMR) spectroscopy. With RasMol, users can rotate, zoom, and manipulate the molecular models to better understand their structure and function [21].PyMOL is a software that provides a range of tools and features that enable users to manipulate molecular models, view them in various representations (e.g., cartoon, surface, sticks), measure distances and angles, create publication-quality images, and conduct structural analyses. Hence PyMOLwas used to gain insights into the structure-function relationships of biomolecules and to aid in the study of protein-ligand interactions [22].The method then conducts docking using the widely-used Autodock Vina program. Through careful optimization, CB-Dock achieved an impressive ~70% success rate for top-ranking docking poses with RMSD within 2 Å from the X-ray pose, outperforming other blind docking tools in benchmark tests. Additionally, CB-Dock provided a 3D visualization ofprotein-ligand interactions of 5FN5 and E2012 of the results and it is freely accessible at CB-Dock website [23].
III. RESULTS AND DISCUSSIONS
In this study, BLASTP was run to check the sequence similarity, the molecular 3D structure of 5FN5 (Human γ-secretase) was visualized and studied using software tools such as RasMol, and Pymol. Next generation sequencing and multiple sequence alignment was achieved through COBALT. Using Pubchem, a potential modulator for 5FN5 was identified. Finally, molecular docking was performed using the CB-Dock.
After opening the SDF file that was downloaded from PubChem, it opened in the RasMol software. In this study, RasMolwas used to visualize the 3D structure of 5FN5 molecule. The molecule was visualized using various commands such as Cartoon, shapely, wireframe, backbone, sticks, ball and stick, and ribbon. The results obtained from RasMol are as follows:\

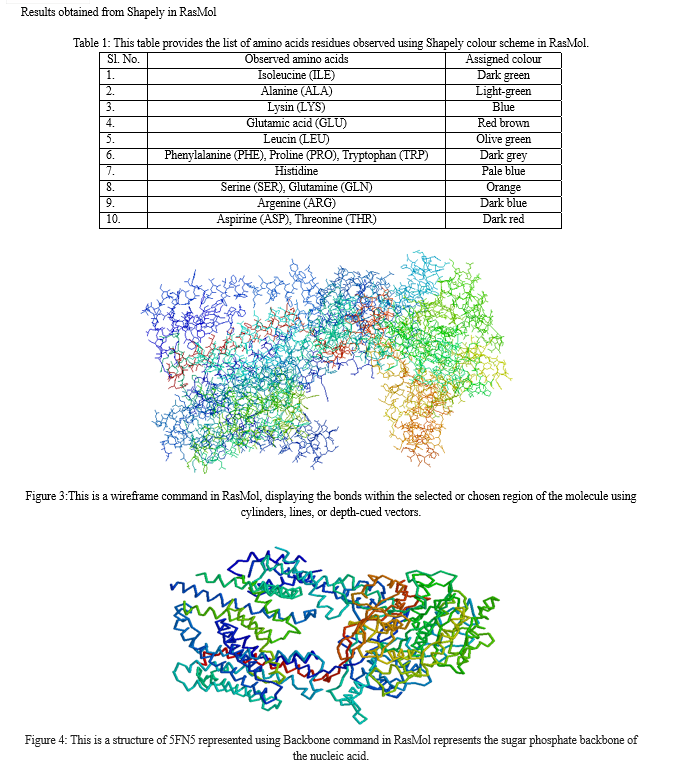
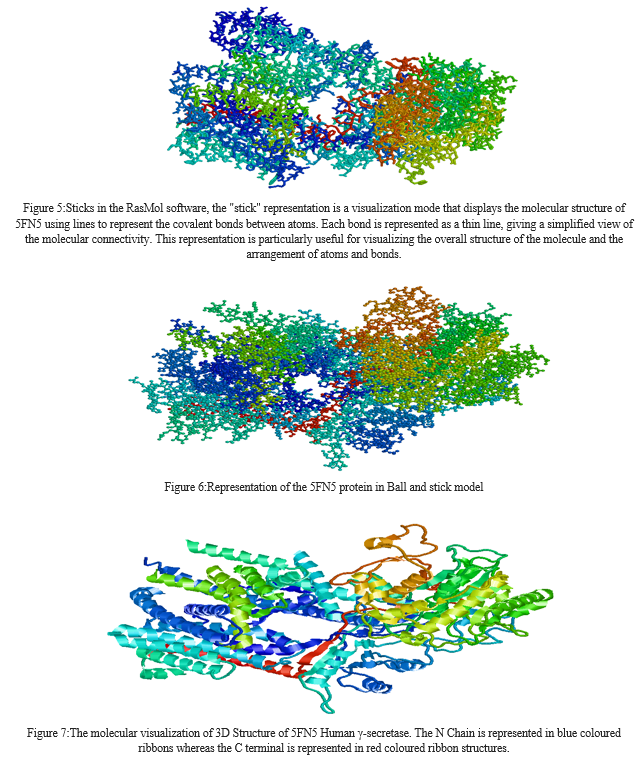

After retrieving the 5FN5 protein sequence from NCBI, it was transformed into FASTA format and used as a query sequence in
BLASTP. BLASTP compares a protein sequence with a database of protein sequences. The BLASTP result displayed a list of
hundreds of sequences in the description that were similar to the query sequence. The E-value in the study of the 5FN5 protein
(Human gamma secretase protein) was zero, indicating that the query and subject sequences were better aligned.Query coverage was 100%, which indicated that the lengths of the query and subject sequences were the same. According to Per.Ident, the query sequence,Nicastrin isoform 1 precursor [Homo sapiens] and KIAA0253 [Homo-sapiens] have a 100 percent similarity, whilst other sequences have a similarity ranging from 89.70 percent to 99.72 percent.
V. RESULTS OF COBALT
COBALT was used to perform the multiple sequence alignment and Next generation sequencing (NGS). COBALT helped to view the alignment information and data of 5FN5 protein from individual sequence. The query sequence was retrieved from NCBI in FASTA format and multiple aligned using COBALT. It does not try to employ all accessible constraints, but rather a highscoring consistent subgroup that can vary as the alignment progresses. The COBALT result emphasized the amino acid residues among the sequences, giving us insight into which amino acid is present in the sequences that are common.
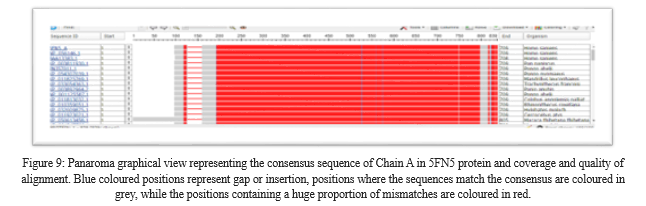


Conclusion
Alzheimer’s Disease is a complex neurodegenerative condition, and the development of effective drugs to treat and cure it has been very challenging. Many drugs have been investigated and tested over the years, but unfortunately, most of them have not resulted in a definitive cure or fully effective treatment for the disease.However, using computational toolkits offers a comprehensive solution for protein sequence analysis, structure visualization, and homology modelling, drug discovery and medical diagnostics.In this research study, Human ?-secterase, 5FN5 was the targeted protein. 5FN5 causes the aberrant cleavage of integral membrane proteins such as notch receptors and APP (?-amyloid precursor protein) leading to Alzheimer’s disease. Thus, various drugs were studied for binding with 5FN5 protein. After docking the various drugs,(3E)-1-[(1S)-1-(4-fluorophenyl)ethyl]-3-[[3-methoxy-4-(4-methylimidazol-1-yl)phenyl ]methyl idene]piperidin-2 (also known as E2012) was found to be the accurate therapeutic drug for 5FN5 protein. From the obtained results using RasMol, BLASTP, PubChem, COBALT, PyMOL, and CB-Dock, and after analyzing the molecular docking results, the structure-based docking images, and template-based docking image displayed better protein–ligand (5FN5 – E2012) interaction and docking table showed significant docking score of –9 which is a good docking score for the binding of E2012 drug ligand to the binding site of 5FN5 protein. Hence, we can conclude that E2012 can be one of the novel compounds with modulatory activity for preventing 5FN5,human gamma secretase that plays a crucial role in Alzheimer’s disease. By providing valuable insights into protein structure-function relationships, this CADD approach can contribute to advancing our understanding of such complex abnormal functioning of proteins in and around the neurons insidethe biological systems and support to the development of new therapeutic strategies for other disorders as well.
References
[1] Breijyeh, Z., &Karaman, R. (2020). Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules, 25(24), 5789. [2] Yiannopoulou, K. G., &Papageorgiou, S. G. (2020). Current and future treatments in Alzheimer disease: an update. Journal of central nervous system disease, 12, 1179573520907397. [3] Zhang, X. X., Tian, Y., Wang, Z. T., Ma, Y. H., Tan, L., & Yu, J. T. (2021). The epidemiology of Alzheimer’s disease modifiable risk factors and prevention. The journal of prevention of Alzheimer\\\'s disease, 8, 313-321. [4] Hardy, J., &Selkoe, D. J. (2002). The amyloid hypothesis of Alzheimer\\\'s disease: progress and problems on the road to therapeutics. science, 297(5580), 353-356. [5] Medeiros, R., Baglietto?Vargas, D., &LaFerla, F. M. (2011). The role of tau in Alzheimer\\\'s disease and related disorders. CNS neuroscience & therapeutics, 17(5), 514-524. [6] Murpy, M., & LeVine III, H. (2010). Alzheimer’s disease and the ?-amyloid peptide. J Alzheimers Dis, 19(1), 311-323. [7] DeTure, M. A., & Dickson, D. W. (2019). The neuropathological diagnosis of Alzheimer’s disease. Molecular neurodegeneration, 14(1), 1-18. [8] Rao, Y. L., Ganaraja, B., Murlimanju, B. V., Joy, T., Krishnamurthy, A., & Agrawal, A. (2022). Hippocampus and its involvement in Alzheimer’s disease: a review. 3 Biotech, 12(2), 55. [9] Neugroschl, J., & Wang, S. (2011). Alzheimer\\\'s disease: diagnosis and treatment across the spectrum of disease severity. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine, 78(4), 596-612. [10] Kaloni, D., & Negi, A. (2019). A review on Alzheimer disease. Int. J. Neurodegener. Dis, 2(10). [11] Ajenikoko, M. K., Ajagbe, A. O., Onigbinde, O. A., Okesina, A. A., & Tijani-Adekilekun, A. (2022). Review of Alzheimer’s disease drugs and their relationship with neuron-glia interaction. IBRO Neuroscience Reports. [12] Golde, T. E. (2022). Disease-modifying therapies for Alzheimer’s disease: More questions than answers. Neurotherapeutics, 19(1), 209-227. [13] Hur, J. Y. (2022). ?-Secretase in Alzheimer’s disease. Experimental & Molecular Medicine, 54(4), 433-446. [14] Golde, T. E., Koo, E. H., Felsenstein, K. M., Osborne, B. A., & Miele, L. (2013). ?-Secretase inhibitors and modulators. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1828(12), 2898-2907. [15] Bai, X. C., Rajendra, E., Yang, G., Shi, Y., &Scheres, S. H. (2015). Sampling the conformational space of the catalytic subunit of human ?-secretase. elife, 4, e11182. [16] Sayers, E. W., Agarwala, R., Bolton, E. E., Brister, J. R., Canese, K., Clark, K., ... &Ostell, J. (2019). Database resources of the national center for biotechnology information. Nucleic acids research, 47(Database issue), D23. [17] Wheeler, D., & Bhagwat, M. (2008). BLAST QuickStart: example-driven web-based BLAST tutorial (pp. 149-175). Humana Press. [18] Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., ... &Bourne, P. E. (2000). The protein data bank. Nucleic acids research, 28(1), 235-242. [19] Chen, J., Anderson, J. B., DeWeese-Scott, C., Fedorova, N. D., Geer, L. Y., He, S., ... & Bryant, S. H. (2003). MMDB: Entrez\\\'s 3D-structure database. Nucleic Acids Research, 31(1), 474-477. [20] Papadopoulos, J. S., &Agarwala, R. (2007). COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics, 23(9), 1073-1079. [21] Bernaerts, K. V., & Hoffmann, A. V. (1997). RasMol as a general tool for the visualization of biomolecules. Computer Applications in the Biosciences: CABIOS, 13(3), 309-312. [22] Schrödinger, L. L. C. (2010). The PyMOL molecular graphics system, version 1.3 r1. [23] Uma kumari, Devanshi Gupta, In silico RNA aptamer drug design and modelling,2022/4, Journal-JETIR,Volume-9, Issue-4, Pages 718-725 [24] Liu, Y., Grimm, M., Dai, W. T., Hou, M. C., Xiao, Z. X., & Cao, Y. (2020). CB-Dock: A web server for cavity detection-guided protein–ligand blind docking. Acta PharmacologicaSinica, 41(1), 138-144.
Copyright
Copyright © 2023 Uma Kumari, Sudhanva Devaprasad Dixit. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET55081
Publish Date : 2023-07-28
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

