Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Nose to Brain Drug Delivery System
Authors: Rohan Pawar, Shubham Pawar
DOI Link: https://doi.org/10.22214/ijraset.2024.59316
Certificate: View Certificate
Abstract
The treatment of brain disorders is particularly challenging due to the presence of a variety of formidable obstacles to deliver drugs selectively and effectively to the brain. Blood-brainbarrier (BBB) constitutes the major obstacle to the uptake of drugs into the brain following systemic administration. Intranosedelivery offers a non-invasive and convenient method to bypass the BBB and delivery of therapeutics directly to the brain. The review discusses the potential of intranoseroute to deliver drugs to the brain, the mechanisms and pathways of direct nose to brain drug transport, the various factors influencing transnosedrug absorption, the conventional and novel intranosedrug delivery systems, the various intranosedrug delivery techniques and devices, and examples of brain drug transport that have been feasible in treating various brain disorders. Moreover, products on the market, investigational drugs, and the author’s perceptions about the prospect of intranosedelivery for treating brain disorders are also been discussed.
Introduction
I. INTRODUCTION
The brain is one of the splendorous examples of God’s creation controlling all the motor or sensory activities in humans and animals. However, the mechanisms that protect it from exogenous molecules also inhibit the entry of medicaments in to the brain, rendering debilitating brain disorders almost untreatable. Blood-brain-barrier represents the strictest barrier to the brain drug delivery inhibiting the ingress of nearly all large-molecule drugs and more than 98% of smallsized therapeutics [1]. BBB is comprised of layers of tightly packed cells at the brain capillary endothelium, the choroid plexus epithelium and the arachnoid membranes, together separating the brain and the cerebrospinal fluid from systemic circulation and resulting in a trans-endothelial electric resistance of 1500-2000 ???? cm2, approximately 100 times than in any part of the body [2-6]. So, there is no paracellular pathway for free exchange of solutes between CSF and blood, and also, pinocytosis is minimal demonstrating minimum transcellular exchange of solutes. Thus, BBB represents the major rate limiting feature in drug delivery to the brain demonstrating maximal permeation of hydrophobic molecules while minimal of hydrophilic [7-9]. Three strategies have been reported to overcome the BBB [1, 10]. First involves drug delivery across the BBB making use of either prodrug approach or carriers like liposomes, nanoparticles, etc. or vectors like receptor-specific monoclonal antibodies, peptides, molecules, etc or vasoactive agents. However, this strategy entails that the drug should possess certain specific characteristics so as to be formulated into prodrugs or nanoparticles and hence, cannot be applied successfully to all the therapeutics. The second trans-cranial approach involves direct delivery to the brain using neurosurgical procedures viz intracerebral implantation, intracerebro ventricular or intracerebral infusion, and convection enhanced diffusion, which have their own specific limitations [1, 10]. As the strategy involves invasive neurosurgical procedures is used for specific applications only like in cancer or neurological pain [11]. The third rapidly budding strategy involves bypassing the BBB using non-invasive intranosedelivery [12]. It is simple, rapid, convenient, amenable for self-administration, reliable and does not require any modification of the therapeutics [13]. The intranoseroute exploits the unique neural connection that the olfactory and the trigeminal nerves provide between the nose and CSF to deliver drugs to the brain. This route can be exploited as a potential alternative drug delivery route for efficient delivery of challenging drugs such as low molecular weight polar compounds, peptides, proteins and large proteins and polysaccharides like vaccines or DNA plasmids. Evidences of nose-to-brain transport have been reported by many scientists round the globe with Illum Lisbeth thoroughly reviewing the possibilities, problems, and solutions of nosedrug delivery [13]. Also, drug absorption across the olfactory region of the nosemucosa provides distinctive feature and better option to preferentially target the drugs to the brain although there are some studies that are contrary [14-16]. Many of the previously abandoned potent centrally acting drugs promise to become successful therapeutic agents via the intranoseroute. The present review discusses the importance of intranoseroute in the treatment of brain diseases/disorders, advantage sand limitations of this delivery route, the mechanisms and pathways of nose-to-brain drug transport across nosemucosa, factors affecting drug delivery across nosemucosa, the various novel drug delivery approaches used to enhance the rate and extent of drug absorption transnasally, intranosedrug delivery techniques and drug delivery devices, applications of intranosedrug delivery in managing brain disorders and the few of the marketed and investigational intranoseformulations.
II. ADVANTAGES OF NOSEDRUG DELIVERY SYSTEM
- Drug degradation is absent.
- Hepatic first – pass metabolism is absent.
- Rapid drug absorption.
- Quick onset of action.
- The bioavailability of larger drug molecules can be improved by means of absorption enhancer or other approach.
- Better nosebioavailability for smaller drug molecules.
- Drugs which can not be absorbed orally may be delivered to the systemic circulation through nosedrug delivery system.
- Convenient route when compared with parenteral route for long term therapy
- Better patient compliance and self medication
- It avoids hepatic first –pass metabolism
III. DISADVANTAGES OF NOSEDRUG DELIVERY SYSTEM
- Stability of drug in-vivo
- Targeting specificity
- Drug irritation and toxicity
- Immunogenecity of proteins
- Drug retention and clearance
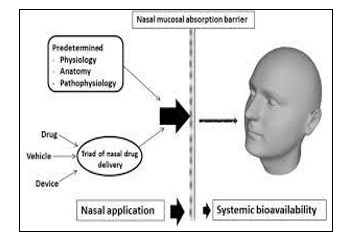
A. Limitations
- Absorption surface area is less when compared to GIT
- High molecular weight of drugs may result in decreased permeability across nosemucosa
- Once the drug administered can not be removed
- Nosecongestion due to cold or allergic condition
- Noseirritation
B. Mechanism of Action
The initial stage of drug absorption from the nosecavity is the drug's passage through the mucus. Mucus readily allows uncharged and tiny particles to flow through. Larger and charged particles, however, might have a harder time crossing. Although several processes have been suggested, the following two have received the most attention. The water route of transport (paracellular route) is involved in the first process of drug absorption. The paracellular pathway is passive and sluggish. The second method involves the drug's transcellular transfer via a lipoidal pathway. Transport of lipophilic drugs—whose rate of transport depends on their lipophilicity—occurs via the reticular pathway..
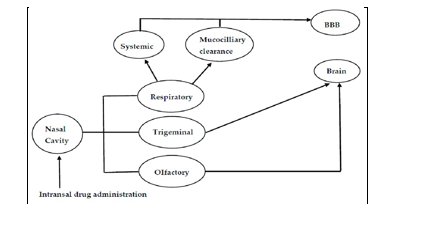
IV. INTRANOSEROUTE FOR BRAIN DRUG DELIVERY
When administered by the intranoseroute, a medication must quickly and efficiently pass through the nosemucosa in order to have a central impact. From a kinetic perspective, the nose is a complicated organ where drug deposition, clearance, and absorption all take place at the same time [17]. Therefore, knowledge of the architecture and physiology of the nosecavity is essential to comprehending the mechanisms and pathways of drug transport to the brain after intranosedelivery.

A. Intranosepathways Of Drug Transport To The Brain
Numerous routes supporting nose-to-brain medication transport have been documented (Fig. 2). Nevertheless, after intranoseadministration, a combination of these channels delivers treatments to the brain; yet, based on the characteristics of the therapeutic, its formulation, and the delivery method employed, one pathway may predominate [26].
B. Neural Pathways
Therapeutics can be delivered from the nose to the brain via a special pathway made possible by the neural connections that the trigeminal and olfactory nerves create between the nosemucosa and the brain [13]. For the purpose of delivering medication to the brain, this neural pathway may use either an extraneuronal/paracellular or an intraneuronal/transcellular pathway, or both. The axonal transport of medications to the various brain regions is a part of the sluggish intraneuronal route. Drugs are delivered directly to the brain through the extraneuronal pathway in a matter of minutes [22, 26, 43].
C. Vascular Pathways
In addition, after being administered via the nose, the medications may enter the brain transnasally via the blood arteries that supply the nosecavity and systemic circulation. Drugs were first administered using the intranoseroute, which involved absorption into the capillary blood vessels beneath the nosemucosa and subsequent delivery to the systemic circulation. The internal and external carotid arteries, as well as the branches of the maxillary and facial arteries, give blood to the highly vascularized nosemucosa [49, 53]. The anterior and posterior ethmoidal arteries, which are minor branches of the ophthalmic artery, supply blood to the olfactory mucosa, while the sphenopalatine artery, which is a branch of the maxillary artery, supplies blood to the respiratory mucosa [54 ]. The respiratory mucosa is the best area for medication absorption into the systemic circulation because it has a higher relative density of blood vessels than the olfactory mucosa. The combination of continuous and fenestrated endothelium in the respiratory region [55, 56] permits the outflow of both small and large mole noseitems for brain medication delivery that are commercially available and in development. cules into the bloodstream and then go to the brain via the blood-brain barrier. This is particularly true for smaller lipophilic medications, which penetrate the blood-brain barrier and enter the bloodstream more readily than larger hydrophilic medications like peptides and proteins. It's also feasible that the medications penetrate the venous blood flow as opposed to dispersing throughout the systemic circulation.
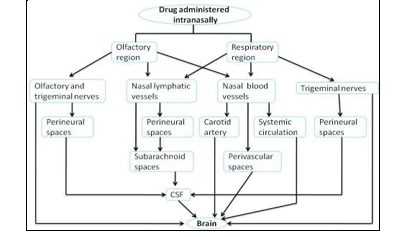
D. Lymphatic Pathways
The idea that CSF is produced in the choroid plexus and then absorbed into the cerebral venous sinuses by arachnoid villi was widely accepted for a number of years. Nonetheless, only a small number of studies over the past 20 years have described the functional and anatomical relationship between the subarachnoid space and the extracranial lymphatics (cervical and nosesubmucosal lymphatics) through the cribriform plate and perineural spaces. [78–81] The nosesubmucosal layer is made up of a thick lymphatic network that connects directly to the subarachnoid region and a dense vascular network that leads to systemic circulation. Through a perineural pathway to the cribriform plate, the nosesubmucosal lymphatics connect directly to the subarachnoid region. The subarachnoid space through the cribriform plate via a perineural pathway. The noselymphatics have been suggested as a possible route for the invasion of multiple pathogens, including S. pneumoniae, N. meningitis, or H. influenzae, which cause bacterial meningitis. They also provide a direct shortcut to the subarachnoid space [82].
E. CSF Pathways
Pathways that connect the noselymphatics, which are essential for CSF circulation and drainage, to the perineural spaces—which include olfactory nerves and the subarachnoid space, which includes CSF—allow access to the CSF and other areas of the brain. Substances secreted by the lateral and fourth ventricles of the four choroid plexi, in particular, cause the generation of CSF. To cushion the brain, the choroid plexi secrete CSF, a secretory fluid [43]. It is reported that tracers injected into the cerebral spinal fluid (CSF) in the subarachnoid space or cerebral ventricles drain into channels that are related to olfactory nerves that traverse the cribriform plate on the underside of the olfactory bulbs. The tracers then travel to the noselymphatic and cervical lymph nodes. system [70–73].As a result, CSF passes between the cribriform plate of the skull, the olfactory submucosa, and the olfactory axon bundles and the nosecavity's roof.
V. FACTORS AFFECTING TRANSNOSEDRUG ABSORPTION
The delivery mechanism and delivery device have a major influence on the drug's deposition and the area where it is deposited because of the unique anatomy and physiology of the nosecavity[83]. The drug being utilized, the therapeutic indication, the patient demographic, and marketing preferences all play a role in the delivery system selection process [84]. Consequently, a multitude of factors impact the absorption of medications transnasally. All these variables, however, are connected with one another; biological factors rely on formulation-related variables, while forulation-related variables rely on drug-related variables. A few of these elements are covered in this section and ought to be considered while researching and creating a novel noseformulation.
A. Biological Factors
To date, a number of approaches have been attempted to alter the anatomical characteristics of the nose in order to improve medication absorption transnasally. These, however, are not appropriate for long-term use since they may cause unfavorable side effects because they affect the nosecavity's normal physiology.
VI. STRUCTURAL CHARACTERISTICS
From the perspective of medication administration, the nose can be physically split into the nosevestibule, atrium/septum, respiratory area, olfactory region, and the nasopharnyx (1).The inferior, middle, and superior tubinates, which are principally in charge of heating and humidifying the air that is inhaled, divide the nosecavity in half along the noseseptum's central axis[10, 21–24]. The keratinized stratified squamous epithelium of the vestibular region has nosehairs to collect and filter airborne particles. From the perspective of nosemedication distribution, it is the least significant. The respiratory mucosa is made up of basal cells, goblet cells, and nonciliated and ciliated columnar cells with hundreds to thousands of microvilli per cell. This is the biggest and has the highest level of vascularity, which is what allows drugs to be absorbed systemically. The olfactory area, which includes the basal cells, supporting cells, and olfactory neural cells, is in charge of CNS medication absorption through the nosemucosa. Therefore, after intranosedelivery, the kind, density, and quantity of cells in the various noseareas affect the absorption of the drug. In order to increase the permeation of these compounds, various authors report using absorption enhancers in conjunction with drugs. These mechanisms may include increasing membrane fluidity, reducing nosemucus viscosity, inhibiting proteolytic or other mucosal enzymes, disrupting tight junctions, increasing paracellular or transcellular transport, or any combination of these. In addition, it has been observed that mucoadhesive dosage forms improve the intranosedelivery of substances [85].
A. Enzymatic Degradation Or Biochemical Features
Drugs administered nasally though avoid extensive metabolism in the gastrointestinal tract and first-pass metabolism in the liver may be susceptible to the enzymes of the nosemucosa presenting a significant barrier to the systemic drug absorption. These include oxidative and conjugative enzymes (e.g., glucuronyl transferase and glutathione transferase), cytochrome P450, carboxy esterase, aldehyde dehydrogenase, carbonic anhydrase, exopeptidases and endopeptidases(e.g., aminopeptidase, carboxypeptidase, trypsin like activities, and cathepsins), etc. [28, 29]. Nosemucus hosts a large number of enzymes such as oxidative and conjugative enzymes, peptidases and proteases which together constitute an enzymatic barrier to the nosedelivery of drugs specially the peptides. These enzymes degrade the drugs within the nosemucosa causing a pseudo-first-pass effect impeding the nosedrug absoption [86, 87]. Noseproteases and peptidases have been implicated in a poor absorption of peptidic drugs,such as calcitonin, insulin, LHRH and desmopressin [88]. However, the noseroute is still being considered superior to the oral delivery of these proteinaceous drugs. Similarly, nasally administered decongestants, alcohols, nicotine and cocaine have been reported to be metabolised by the noseP450-dependent monoxygenase [36]. Various approaches such as the use of protease and peptidase inhibitors like bacitracin, amastatin, boroleucin and puromycin [89,90], have been reported to improve the noseabsorption of LHRH peptides [91], leucinenkephalin [92] and human growth hormones [93]. Prodrugs have also been reported to increase the nosestability and permeation of compounds like esters of steroids (e.g. beclomethasone dipropionate monohydrate), cromoglycic acid (charged prodrug), an some peptides and amino acids (e.g. desmopressin acetate and L-tyrosine) [94, 95].
B. Blood Supply And Neuronal Regulation
The nosecavity is highly vascularized due to the presence of venous sinusoids and arteriovenous anastomosis important for the heating and humidification of the inspired air and noseresistance. Nosecycles of congestion (increased blood supply) on parasympathetic stimulation [96, 97] and relaxation (decreased blood supply) on sympathetic stimulation [97, 98] regulate the amount of drug absorbed, respectively [99].
Based on a study in dogs (anaesthetized with penobarbitone) electrical stimulation of the parasympathetic nerves innervating the nosemucosa resulted in an increased drug permeation due to an increase in noseblood flow and nosesecretion.
C. Transporters and Efflux Systems
An active research area in the field of intranosedrug delivery is the study of the various transporter systems present in the nosetissue and their effects on the absorption of drugs into systemic circulation and/or brain. Presently, multidrug resistance transporters have been identified in the human noserespiratory and olfactory mucosa, which may be influence the transport of a wide variety of hydrophobic and amphiphilic drugs transnasally [103]. P-gp, an ATP dependent efflux transporter exists in the apical area of ciliated epithelial cells and in the submucosal vessels of the human olfactory region [104]. Several studies demonstrate that Pgp plays an important role in preventing active influx of drugs from the nosemucosa systemic circulation and/or brain [104-106].
D. Nosesecretions
The various mucosal and submucosal glands secrete nosemucus that forms a continuous layer of 5 ????m on the nosemucosa. Approximately 1.5-2 l ml of mucus is produced daily and exists as a double layer consisting of a watery hypophase, adjacent to the epithelial surface, in which the cilia beat and a more viscous gel like epiphase which is moved forward by the beating cilia [24]. Both the composition and the viscosity of nosesecretions affect intranosedrug absorption with mucus composition affecting the drug solubility while viscosity altering the time of contact of the drug with the nosemucosa. 90% water, 2% mucus, 1% salts, 1% proteins (mostly albumin, immunoglobulins, lysozyme, lactoferrin, etc.), and 1% lipids make up the nosesecretions [22, 24, 27]. In order for a medication to penetrate the nosemucosa and dissolve in nosesecretions, it must possess the correct physicochemical characteristics. It has been demonstrated that administering water-soluble equivalents of experimental medications through the noseroute improves drug absorption [107]. Changes in the viscosity of nosemucus, either in the hypophase or epiphase, have been shown to impact ciliary beating, which in turn impacts the duration of drug interaction with the nosemucosa and, ultimately, the absorption of the drug [108].
E. Nosecycle
Numerous investigations have demonstrated that nosecycle frequency and nosemucus secretion and clearance rates are influenced by circadian rhythms. There have been reports that the nosecycle occurs more frequently during the day than it does at night and in the early morning. Similar to this, a number of studies show that nosesecretion production and clearance rates decrease at night, which has an impact on nosemedication absorption [109].
F. pH of the Nosesecretions
The pH of the nosesecretions varies between 5.5-6.5 in adults and 5.0-7.0 in infants. A drug will be absorbed better when the nosemucus pH is lower than the drug’s pKa as the drug will be present predominantly in an unionized form [94]. Thus, a change in the pH of nosesecretions can affect the drug ionization altering the amount of drug absorbed transnasally. Since, the pH of the nosemucus can alter the pH of the formulation and viceversa, the pH of a formulation should ideally be 4.5 to 6.5 with adequate buffering capacity.
VII. MUCOCILIARY CLEARANCE
The nosemucociliary clearance is an important clearance mechanism to remove foreign particles such as dust, allergens and bacteria, trapped on the mucus blanket during inhalation. The drug absorption is influenced by the contact time between the drug and the nosemucosa. The mucociliary clearance is inversely related to the residence (contact) time and thus, inversely proportional to the absorption of drugs administered [110]. Various strategies have been used to prolong the residence time of the drug in the nosecavity such as using bioadhesive polymers like chitosan or polycarbophils or increasing the viscosity of the formulation. Nosemucociliary clearance is also stimulated or inhibited by drugs, excipients, preservatives and / or absorption enhancers and thus affects the nosedrug absorption [111, 112].
VIII. PATHOLOGICAL CONDITIONS
An essential mechanism for clearing away unwanted particles— such as dust, allergens, and bacteriathat become lodged on the mucus blanket during inhalatio n is the nosemucociliary clearanceThe duration of contact between the medicine and the nose mucosa affects how well the drug is absorbed.The mucociliary clearance is inversely proporti onal to the absorption of given medications since it is inversely connected to the residence (c ontact) time [110].
Many tactics, such as adding more viscosity to the formulation or employi ng bioadhesive polymers like chitosan or polycarbophils, have been tried to extend the durati on of the drug's residence in the nosecavity.Drugs, excipients, preservatives, and/or absorptio n enhancers can also stimulate or inhibit nosemucociliary clearance, which might impact nose medication absorption [1111Nosemucosal irritation, hyper- or hyposecretions, and mucociliary dysfunction are frequently linked to local noseinfections such the common cold, rhinitis, and nosepolyposis, which can affect transnosedrug absorption [113]. Numerous medications have been evaluated and categorized as either cilio-inhibitory or cilio-friendly, providing an invaluable tool for the development of safe nosemedications [114].
A. Environmental Factors
Temperatures nearby 24°C cause a moderate reduction in the rate of mucociliary clearance.
However, linear increase in ciliary beat frequency occurs with increase in temperature [115].
B. Formulation Related Factors
A noseformulation usually consist of the drug, a vehicle, and the excipients. The physicochemical properties of drugas well as the formulation are imperative from the formulation design point of view.
IX. PHYSICOCHEMICAL PROPERTIES OF DRUGS
The rate and extent of drug absorption post intranoseadministration depends on the following physicochemical characteristics of the drug
A. Lipophilicity
Using a variety of chemicals transnasally, a linear association between lipophilicity and drug penetration has been established. In contrast to the hydrophilic molecule metoprolol, lipophilic medicines such as alprenolol and propranolol are readily absorbed across nosemucosa, despite the nosemucosa having certain hydrophilic characteristics. Lipophilic substances easily penetrate the nosemucosa through the transcellular pathway, whereby they diffuse into the cytoplasm and partition into the lipid (bilayer) of the cell membrane [116, 117]. In animal models, it has been observed that certain lipophilic medications, including progesterone, naloxone, buprenorphine, barbiturates, testosterone, and 17-ethinyloestradiol, are nearly entirely absorbed transnasally.
B. Chemical Form
When it comes to a drug's ability to penetrate mucosal surfaces, its molecular form is crucial. Huang et al. investigated how L-Tyrosine's structural changes affected its transnoseabsorption, finding that L-Tyrosine's carboxylic acid ester absorbed considerably more than L-Tyrosine [118]. Therefore, changing the drug's chemical makeup to make it a salt or an ester can change how well it is absorbed..
C. Polymorphism
It is well established that polymorphism influences a drug's solubility, rate of dissolution, and ability to pass through biological membranes to be absorbed [119, 120]. Therefore, it is preferable to research the purity and polymorphic stability of medications, particularly for nosepowders and/or solutions.
D. Solubility And Dissolution Rate
Transmucosal absorption from nosepowders and solutions is dependent on the drug's solubility and rate of dissolution. In order for the particles to be absorbed transnasally, they must first dissolve on the nosemucosa. No absorption occurs if a medication is eliminated or stays in the form of particles..
E. Molecular Weight
A drug's transnosepenetration is determined by both its molecular weight and lipophilicity working together. It has been found that the transnosepenetration of medicines and their molecular weight up to 300 daltons are inversely related. Nevertheless, unless absorption enhancers are used, absorption dramatically drops if the molecular weight exceeds 1,000 daltons [121]. Whereas hydrophilic molecules show an inverse relationship, lipophilic compounds show a direct link between the molecular weight and drug penetration.
According to research by Yamamto et al. [123] and Fisher et al. [122], medicines smaller than 300 daltons primarily permeate through paracellular mechanisms, with their physicochemical qualities having little effect. But for medications with molecular weights greater than or equal to weight greater than 200 Da
F. Partition Coefficient and PKA
Unionized species are absorbed more readily than ionized species, as indicated by the pH partition hypothesis, and this also holds true for medication absorption through the nose. A quantitative link between the noseabsorption constant and the partition coefficient has been demonstrated by Jiang et al. [124].However, it has been shown that the pH of the surrounding environment can affect mucosal absorption for weak acids or bases. It has been noted that the degree of ionization of substances such as aminopyrine and salicylic acid greatly influences their nosepenetration. For aminopyrine, there were significant variations in the absorption rate with increasing pH, but not for salicylic acid . The authors proposed that salicylic acid might have a separate transport mechanism in addition to the lipoidal system [125]. Even at 99.9% ionization (pH 7.19) 13% absorption was noted for in unionized form (pH 2.5, 44%) [94].Additionally, it was determined that the unionized species' rate of absorption was four times faster than the ionized species' rate. As a result, the partition coefficient is shown in the results to be a significant factor controlling nosemedication absorption.
G. Shape
Shape is also important. Linear molecules have lesser absorption than cyclic-shaped molecules [126].
H. Physicochemical Properties of Formulation
The following physicochemical properties of the formulation determine the rate and extent of drug absorption following intranoseadministration.
I. PH and Mucosal Irritancy
By changing the degree of drug ionization, the nosecavity and the formulation's pH both have an impact on a medicine's ability to permeate through the nosemembrane. To prevent noseirritation, the formulation's pH should be adjusted to be close to the physiological pH of the nose, which is between 4.5 and 6.5 [127]. Since lysozymes in nosesecretions are active at acidic pH, this pH range also inhibits the growth of germs. Additionally, since medications are absorbed in the unionized state, it ensures effective transmucosal permeation for weakly acidic or basic pharmaceuticals.
J. Buffer Capacity
Typically, noseformulations are delivered in quantities between 25 and 300 μL [10]. As a result, nosesecretions may change the formulation's pH when delivered. The concentration of unionized medication that is available for absorption is subsequently impacted by this. Therefore, to maintain its pH in-situ, a sufficient formulation buffer capacity could be needed.
K. Osmolarity
Typically, noseformulations are delivered in quantities between 25 and 300 μL [10]. As a result, nosesecretions may change the formulation's pH when delivered. The concentration of unionized medication that is available for absorption is subsequently impacted by this. Therefore, to maintain its pH in-situ, a sufficient formulation buffer capacity could be needed.
L. Viscosity
According to a study by Jansson et al., increasing the formulation's viscosity lengthens the medication's contact time with the nosemucosa and, consequently, the time it takes for the drug to permeate [131]. Viscose formulations also change the way medications are transnasally absorbed by interfering with typical physiological processes including ciliary beating and mucociliary clearance.
According to a study by Suzuki et al., hydroxypropyl cellulose was useful in this situation for increasing the absorption of low molecular weight medicines, but it had no such effect on high molecular weight peptides [132]. It is frequently advised by safety experts to combine "generally regarded as safe" (GRAS) carriers.
X. DRUG DISTRIBUTION
One major aspect influencing how well drugs are absorbed in the nosecavity is their distribution. This distribution is influenced by the method of medication administration and the posture used during administration, which can help ascertain the degree of drug absorption. Particle accumulation in the nose is correlated with a person's noseresistance to airflow [133]. Almost 90% of the particles with an aerodynamic size of 10–20 mm are deposited on the nosemucosa during nosebreathing [134].
A. Dosage Form
The transnosedrug absorption is also influenced by the kind of nosedose form that is used. Even though nosedrops are the most straightforward and practical dosage form, they are unable to deliver a precise volume of formulation, which might lead to overdosing [135]. Furthermore, nosedrops can also cause anterior leakage and postnosedrip. Sprays with solutions and suspensions are better than those with particles because powders can irritate mucosal surfaces by drying up the mucous membrane [136]. Metered-dose gel devices that precisely administer medication formulation have been developed recently. Gels localize the formulation within the nosecavity for an extended amount of time by slowing down the formulation's fast noseoutflow [137]. There hasn't been much research on the application of emulsions [138] and ointments [139] as noseformulations. For nosedelivery, specialized methods have also been documented, including liposomes, proliposomes, films, microemulsions [140], microspheres (using chitosan, carbopol 934P, and lactose [140], and niosomes). These allow for a closer and longer period of time for the drug to come into intimate touch with the nosemucosa, increasing the likelihood of drug penetration.
B. Formulation Excipients
Noseformulations contain a wide range of excipients, including antioxidants, solubilizers, and preservatives. Antioxidants, preservatives, humectants, flavorings, and taste masking agents are not predicted to change nosedrug absorption, despite the fact that they cause a number of noseirritations [36].
C. Solubilizers
For nosesolutions, a drug's water solubility is frequently a constraint. Drug solubility can be increased by using conventional solvents or co-solvents like glycols, tiny amounts of alcohol, Transcutol® (diethylene glycol monoethyl ether), medium chain glycerides, and Labrasol® (saturated polyglycolyzed C8-C10 glyceride). Additional choices include combining lipophilic absorption enhancers [147, 148] with surfactants or cyclodextrins, such as hydroxypropyl-beta-cyclodextrin, which operate as biocompatible solubilizers [144, 145] and stabilizers [146]. In these situations, it is important to assess how the solubilizers affect noseirritancy..
D. Preservatives
Because most noseformulations are water-based, preservatives are needed to stop microorganisms from growing. Among the preservatives that are frequently employed in noseformulations are parabens, benzalkonium chloride, phenyl ethyl alcohol, EDTA, and benzyl alcohol [149, 150].
E. Humectants
Mucous membrane dryness and crusts are associated with a number of chronic and allergy disorders. Among other excipients, antioxidants and preservatives have the potential to irritate the nose, particularly when taken in excessive amounts. Intranosemoisture levels must be adequate to avoid dehydration [151]. Humectants include things like sorbitol, mannitol, and glycerin.
F. Drug Concentration, Required Dose, And Dose Volume
The effectiveness of the nosedelivery system is influenced by three interrelated parameters: drug concentration, dosage, and administration volume. In noseperfusion tests, it has been demonstrated that noseabsorption of L-tyrosine increases with drug concentration.
However, aminopyrine was found to absorb as a function of concentration at a constant rate in another investigation [125]. There is a 25–150 ?l volume limit on what can be administered to the nosecavity. Various methods have been investigated to make good use of this volume, such as the use of gelling, viscofying, or solubilizers [152, 153].
G. Role Of Absorption Enhancers
The choice of absorption enhancers is determined by how they affect nosephysiological function and whether or not they are accepted by regulatory bodies. Absorption enhancers change the physicochemical characteristics of the medication, such as its partition coefficient, solubility, etc., or they modify the structure of the nosemucosa to facilitate drug penetration. When a drug has large molecular size, poor membrane permeability, lacks lipophilicity, and is susceptible to enzymatic degradation, absorption enhancers may be necessary [154, 117, 155, 156].
H. Delivery Device Related Factors
Different types of devices are used to deliver formulations intranasally. Both the size and the site and pattern of deposition affect the transnosepermeation of drugs.
I. Size Of The Droplet Or Powder
The device's size and shape determine the size of the droplet that is created. The particles will be expelled if they are less than 0.5 μm and will be deposited in the upper respiratory tract if they are smaller than 10 μm. Smaller particles or droplets, ranging in size from 5 to 7 μm, will be held in the nosecavity and eventually enter through [118].
J. Site And Pattern Of Deposition
The site and pattern of drug deposition is affected by the formulation composition, the dosage form (liquid, viscous, semisolid, solid), the delivery device used, the design of actuators and adapters, and the administration technique [83]. The permeability of the deposition site and the area of nosecavity exposed affect the drug absorption [157]. These factors also determine the retention time of the drug in the nosecavity.
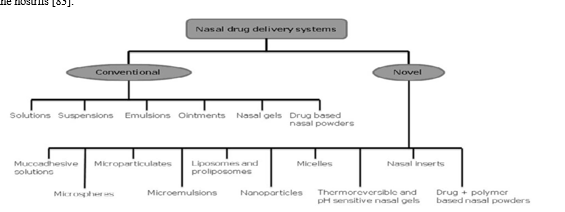
K. Intranoseformulations For Brain Drug Delivery
Because the nosecavity is divided into a ciliated posterior area and a non-ciliated anterior section, the location of deposition has a crucial role in mucociliary clearance, which in turn controls residence, which in turn controls medication absorption of the administered formulation. The distribution mechanism and delivery equipment play a major role in the deposition and deposition. This section discusses several dose forms (Fig. 3) and how they are applied to deliver the medications to the central nervous system after intranosedrug delivery.
XI. LIQUID DOSAGE FORMS
Liquid dosage forms either in form of soluble, suspended or colloidal systems are normally used for formulating nosedelivery systems.
A. Nosedrops
One of the easiest and most practical noseadministration devices ever created is nosedrops. These formulations might be suspension-based or solution-based. This system's primary drawback is its lack of dose precision, which means nosedrops might not be appropriate for goods that are prescribed. According to reports, nosedrops work better than nosesprays at depositing human serum albumin in the nostrils [83].
B. Nosesprays
Both solution and suspension formulations can be formulated into nosesprays. Due to the availability of metered dose pumps and actuators, a nosespray can deliver an exact dose anywhere from 25 to 200 ????L [100, 101, 133]. The particle size and morphology (for suspensions) of the drug and viscosity of the formulation determine the choice of pump and actuator assembly.
XII. NOSEEMULSIONS AND MICROEMULSIONS, LIPOSOMES AND NANOPARTICLES
Although intranoseemulsions have not been studied extensively [140], a large number of data exist to demonstrate the efficacy of intranosemicroemulsions [158-161]. The large surface area available for absorption with microemulsions depicts an advantage over emulsions. Noseemulsions and microemulsions offer the advantages for local application mainly due to their viscosity. One of the major disadvantages is poor patient acceptability. The physical stability of emulsion formulations and precise delivery are some of the main formulation issues. Nanoparticles may ensure an improved transnosedrug delivery to the brain since they are able to protect the encapsulated drug from biological and/or chemical degradation, and extracellular transport by P-gp efflux proteins [162]. This eventually increases the brain concentrations of the drug. Their small diameter potentially allows nanoparticles to be transported transcellularly via the various endocytic pathways through the olfactory neurones to the brain [162]. Surface modification of the nanoparticles can also be tried to achieve targeted nose-to-brain drug delivery. These can be applied both in suspension or powder form.
XIII. SEMI-SOLID DOSAGE FORMS
Semi-solid systems include nosegels, ointments and liquid systems containing temperature or pH sensitive polymers that gel at respective physiological temperature or pH of the nosecavity. These systems ensure longer residence time within the nosemucosa due to their semi-solid consistency.
A. Nosegels
Nosegels are thickened solutions or suspensions of drug in a highly viscous polymer base. Until the recent development of precise dosing devices, there was not much interest in this system. The advantages of a nosegel include longer residence time with in the nosecavity due to its high viscosity, minimised drug wastage due to reduced post-nosedripping and anterior leakage, reduction of taste impact due to reduced post-nosedripping, reduction of irritation by using soothing/emollient excipients, and better drug absorption by offering intimate contact between the drug and the nosemucosa. Vitamin B12 and apomorphine gels have been successfully employed to achieve desired therapeutic concentrations following noseadministration [163]. Thermoreversible gels using temperature sensitive polymers like poloxamers and pH sensitive gels employing pH sensitive polymers like polycarbophils have also been reported and offer advantage of more accurate dosing over conventional gels [160, 164-166].
B. Solid Dosage Forms
Solid dosage forms though not very popular with intranosedrug delivery are more suitable for pulmonary drug delivery and similar applications. However, these systems pose the problem of mucosal irritation by drying of the nosemucosa.
C. Nosepowders
Powder dosage forms may be developed if solution and suspension dosage forms cannot be developed, mainly due to lack of drug stability. The advantages of a nosepowder dosage form are the absence of preservative and superior stability of the drug in the formulation. However, the suitability of the powder formulation is dependent on the solubility, particle size, aerodynamic properties and noseirritancy of the active drug and/or excipients. An additional advantage of this system is local application of drug, but nosemucosa irritancy and metered dose delivery are some of the challenges for formulation scientists and device manufacturers who are interested in powder dosage forms [167]. Apart from plain drug various delivery systems like, microspheres, nanoparticles, liposomes, etc can be formulated as nosepowders.
XIV. NOVEL FORMULATION APPROACHES FOR INTRANOSEDRUG DELIVERY
In order to formulate a noseformulation with desirable performance and commercial attributes, the drug properties, and understood from the early stages of product development. It is advisable to focus on maximizing the residence time and ensuring efficient absorption of drug.
A. Mucoadhesive Solutions
Mucoadhesive solutions comprising mucoadhesive polymers like chitosan, cellulose polymers, polycarbophils, poloxamers, etc. have been reported to enhance drug permeation transnasally [85]. These systems being viscous and mucoadhesive provide longer residence time for better drug absorption. Illum et al. have reported an enhancement in the absorption of insulin across the nosemucosa of rat and sheep using cationic chitosan based nosesolution [168]. Numerous studies have demonstrated that chitosan and their derivatives are effective and safe absorption enhancers to
improve mucosa delivery of hydrophilic macromolecules such as peptides and protein drugs [168, 169]. However, these systems suffer from post-nosedripping and anterior leakage when compared to gels or powder formulations.
B. Microspheres
Microspheres, including mucoadhesive microspheres, are novel systems that are becoming increasingly popular with nosedrug delivery. Microspheres may provide prolonged contact with the nosemucosa enhancing the rate and extent of drug absorption [85]. Microspheres or for noseapplications are usually prepared using biocompatible materials, such as starch, albumin, dextran, hyaluronic acid, chitosan and gelatine, hydroxypropyl methylcellulose, carbopol 934P and various combinations of these polymers [170-172]. These polymers on absorbing nosesecretions form a gel-like layer which is slowly cleared from the nosecavity. However, the toxicity of the polymer on the nosemucosa cells should be critically evaluated. Starch microspheres are most frequently used nosedelivery systems and have been successfully tried for insulin, gentamicin, human growth hormone, metoclopramide and desmopressin [173]. Starch microspheres cause drying of the nosemucosal surface due to uptake of moisture by the microspheres. This results in reversible “shrinkage” of the cells, providing a temporary physical separation of the tight (intercellular) junctions that increases the absorption of drugs [174, 176]. Illum et al. studied the absorption of insulin from bioadhesive DEAEdextran microspheres [176]. Shaji et al. studied the brain delivery of clonazepam from gelatin-chitosan based nosemucoadhesive microspheres in rats [177]. Paolo et al. Have reported nosechitosan microspheres for improved and prolong delivery of rokitamycin to the brain for treating granulomatousnamoebic encephalitis [178].
C. Microparticulates
Microparticulates similar to microspheres constitute an efficient dosage form for the in situ gelling nosedrug delivery. Lim et al. examined the application of previously characterized microparticles composed of hyaluronan and chitosan hydroglutamate as well as novel microparticles consisting of both polymers to improve the nosedelivery of gentamycin [179]. The rabbit bioavialabilities of gentamycin incorporated in hyaluronan, chitosan hydroglutamate and hyaluronan or chitosan hydroglutamate microparticles were increased 23-, 31- and 42-folds respectively as compared to the control intranosesolution of gentamycin. This indicated that the test microparticles were retained for a longer period of time on the rabbit nosemucosa, also supported by the frog palate mucoadhesion studies, thereby improving the drug absorption. The higher bioavailabilities with chitosan hydroglutamate based formulations (chitosan hydroglutamate and hyaluronan/chitosan hydroglutamate) suggested the penetration-enhancing effects of the polymer chitosan hydroglutamate. Krauland, et al. have developed a microparticulate delivery system based on a thiolated chitosan conjugate for the noseapplication of peptides [180]. In this study, insulin was used as a model peptide and they observed that microparticles comprising chitosan-4thiobutylamidine and reduced glutathione seem to represent a useful formulation for the noseadministration of peptides.
D. Microemulsions
Microemulsions are optically isotropic and thermodynamically stable multicomponent fluids composed of oil, water and surfactant. Of the various dosage forms used intranasally microemulsions offer several advantages like high solubilization of lipophilic drugs, thermodynamic stability, easy to prepare and handle, stabilization of hydrolytically susceptible compounds and provide large surface area for better drug absorption. A mucoadhesive microemulsion, consisting of polymers like carbomers or chitosan, in addition to the advantages of a microemulsion will provide longer residence time in the nosecavity, depicting rapid and complete absorption of drugs. Misra et al. have reported an enhanced transport of various drugs to the brain across nosecavity using mucoadhesive microemulsions for the management of various brain or brain associated disorders [34, 160,161, 164]. Bajaj et al. developed some nanoemulsion and gel formulations of rizatriptan benzoate for the treatment of migraine [181]. Li et al. and Zhang et al. have reported better transnoseabsorption of diazepam and nimodipine respectively using microemulsion systems.
XV. LIPOSOMES AND PROLIPOSOMES
Liposomes and proliposomes have been delivered by various routes. In a study on rats by Wattanathorn et al. Intranoseliposomes containing quercetin decreased anxietylike behavior and increased spatial memory [182]. US Patent 6342478 describes a nosemicellar or liposomal preparation for the delivery of fibroblast growth factor to the brain [183]. Vyas et al. have reported multilamellar liposomes for intranosedelivery of nifedipine [184]. Charged components, stearylamine, dicetyl phosphate and some fusogenic/ bioadhesive material were also incorporated into the liposomes. Positively charged liposomes possessed maximum bioadhesion prolonging the residence time within the nosecavity thereby improving the bioavailability. Free flowing proliposomes
containing propranolol hydrochloride were prepared by Shim et al. and evaluated their potential for transnosedelivery of propranolol to sustain its plasma concentration [185].
A. Nanoparticles
Polymeric nanoparticles are efficient carriers for the transnoseabsorption of drugs and proteins. Illum et al. Demonstrated that chitosan based nanoparticles can enhance nose-tobrain delivery of drugs compared to equivalent drug solutions formulations due to the protection of the drug from degradation and/or efflux back into the nosecavity [162]. They have also reviewed the various transport pathways and future strategies for delivering drugs across nosemucosa in the form of nanoparticles. Tang et al. have reported estradiol containing chitosan nanoparticles for improved noseabsorption and brain targeting [186]. Chitosan nanoparticles fo nose to brain delivery of a piperidine cholinesterase inhibitor have also been reported by Ali et al. [187]. The potential of low molecular weight chitosan as carriers for nosevaccine delivery was evaluated by Alonso et al. In mice [188]. Vyas et al. evaluated the in vivo efficacy of plasmid DNA loaded chitosan nanoparticles for nosemucosal immunization against hepatitis B and demonstrated intranoseadministration as a potential route for vaccine delivery [189]. Alonso et al. have demonstrated PEG-PLA nanoparticles as potential nosecarriers for drug/vaccine administration [190]. Jiskoot et al. evaluated the potential of N-trimethyl chitosan (TMC) nanoparticles as a carrier system for the nosedelivery of proteins [191]. They postulated that FITC-albumin-associated TMC nanoparticles successfully transported ovalbumin across the nosemucosa. Gao et al. successfully demonstrated brain uptake following intranoseadministration of Lectinconjugated PEG-PLA nanoparticles [192].
B. Thermoreversible and PH-Sensitive Gels
Thermoreversible nosegels comprised of temperature sensitive polymers like poloxamers and pH-sensitive gels consisting pH sensitive polymers like polycarbophils have been reported to enhance drug permeation transnasally. A lidocaine hydrochloride nosegel have been reported by Xin- Guo et al. using hydroxypropyl methyl cellulose (HPMC) as base material [193]. Mahadik et al. have reported a pluronic PF 127 based thermoreversible nosegel of Vitamin B12. In situ gelling systems based on temperature-dependent phase transition containing pheniramine and phenylephrine were developed using combination of Poloxamers, different cellulose (HPMC) and xanthan gum have been reported by Mehta et al. [194]. Murthy et al. developed a thermoreversible- mucoadhesive gel comprising thermoreversible polymer Pluronic F127 (PF127) and mucoadhesive polymer Carbopol 934P (C934P) for intranosedelivery of sumatriptan [165].
C. Micelles
Micelles are formed by the self-assembly of amphiphilic block copolymers in aqueous solutions and are of great interest with respect to drug delivery applications [195]. The drugs are physically entrapped in the core of the block copolymeric micelles and transported at concentrations that can exceed their intrinsic water- solubility. The hydrophilic blocks also form hydrogen bonds with the aqueous surroundings to form a tight shell around the micellar core protecting the contents of the hydrophobic core effectively from hydrolysis and enzymatic degradation. Mitra et al. have reported that mixed micelles of bile salts and fatty acid have a synergistic effect on the noseabsorption of peptides [196]. They found that maximal noseabsorption enhancement of [D-Arg] kyotorphinhas was observed with mixed micelles of sodium glycocholateand linoleicacid than that with glycocholatealone.
A similar study was performed by Tengamnuay et al. which documented that noseabsorption of insulin in the presence of sodium glycocholate and linoleic acid increased relative than with sodium glycocholate or linoleic acid alone [197]. Micellar nanocarriers have been developed by Patravale et al. as potential carriers for nose-to-brain delivery of zolmitriptan to treat migraine [198].
D. Noseinserts
The noseinserts serve as a novel, new, bioadhesive, solid dosage form for the prolonged systemic drug delivery via the noseroute [199].
The principle involves imbibitions of the nosefluid from the mucosa after administration and to form a gel in the nosecavity to avoid noseirritation. The resulting gel adheres to the mucosa due to its bioadhesive properties, acting as release controlling matrix allowing sustained drug delivery. Due to dissolution of the gel and/or mucociliary clearance, there is no need for the removal of the insert mechanically after it is depleted of drug. The in-situ gelling noseinserts are usually prepared by lyophilisation of aqueous solutions of drug, polymer as carrier and other excipients if required. The sponge-like structure of in-situ gelling noseinserts is an important parameter to ensure rapid hydration and gelation of the inserts at the nosemucosa. The drug release from noseinserts is a complex phenomenon of water penetration, relaxation of the polymer chains, swelling and spreading of the insert, dissolution of the water-soluble polymer and drug, interactions of the drug and carrier, and have reported hydrophilic polymer based noseinserts for the delivery of influenza vaccine [200].
XVI. NOSEDRUG DELIVERY TECHNIQUES
Differences in the administration techniques employed can affect deposition within the noseepithelium and delivery along the various pathways to the brain. The various techniques employed for nosedrug delivery are discussed inthis section
A. Snorting
This method used from several ages by the drug addicted made the basis for the intranosedrug delivery. Snorting involves sniffing a highly concentrated powder form of a drug such as cocaine or heroin and rapidly into the nostril. This deposits the drug powder onto the nosemucosa and rapidly transfers it into the circulation and brain. However, the technique requires an experienced and cooperative user for effective drug delivery across the nose.
B. Drug Delivery Using A Syringe Or Dropper
A second method of intranosedrug delivery involves dripping a few drops of the solubilized medication (liquid form) into the nose using a syringe or dropper and allowing it to run down onto the nosemucosa. The syringe or dropper acts as the measuring/dosing device The efficacy of the nosedropper techniques are highly variable although some authors find these to be an effective method of nosedrug delivery [201-203]. The limitations of the technique are post nosedripping and anterior leakage resulting in non-uniform dosing of medication [201,204]. However, this method has been used in many researches and has been found to be an effective method of delivering adequate doses of medication to patients.
C. Sprayed Or Atomized Medication Delivery
Intranosedrug delivery in spray or atomized form is a most recent technique adopted by the pharmaceutical industry due to improved usability as well as improved bioavailability. The technique combines a method of measuring a unit dose of medication either via a syringe or unit dose pump, with a spray tip distributing the medication into fine particles as it is being sprayed into the nose. The method of delivery results in a broader distribution of drug across the nosemucosa resulting in an increased bioavailability of the drug. [201, 204-208] Furthermore, the usability issue makes this nosespraying of medications far easier to employ as it is patient compliant and takes only a second to administer the dose intranasally. Since, the drug formulation is sprayed or atomized as a mist post nosedripping and anterior leakage are minimal. For all these reasons, most pharmaceutical nosemedications are now packaged with a spray applicator rather than a dropper. In addition, syringe driven and pump driven spraying devices (atomizers) now exist for delivery of a variety of generic nosemedications.
D. Nosedelivery Devices
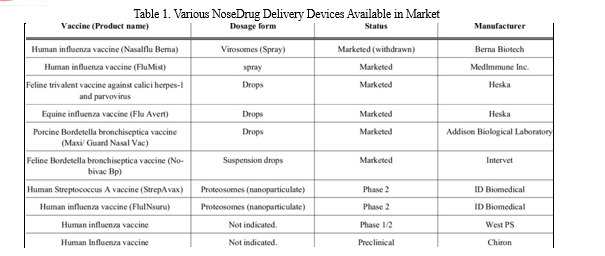
A successful noseformulation program involves detailed consideration of the interactions between formulation composition, device design, delivery system and the patient’s pathological condition. Various delivery devices are available for intranosedrug administration Fig. (4). Devices vary in accuracy of delivery, dose reproducibility, cost, and ease of use. Currently, metered-dose systems provide the greatest dose accuracy and reproducibility. Differences also exist in force of delivery, emitted droplet size, and spray patterns. If a noseformulation is delivered to the target site of absorption (turbinates), benefits can be gained from increased absorption and/or decreased dosage requirements. Delivery devices are important not only for delivering medication, but also for providing an appropriate environment for formulation storage which includes protection from microbial contamination and chemical degradation. The device and formulation should be compatible so as to avoid potential leaching or adsorption. Table 1 describes the characteristics of the individual device used to deliver drugs intranasally [209-214].
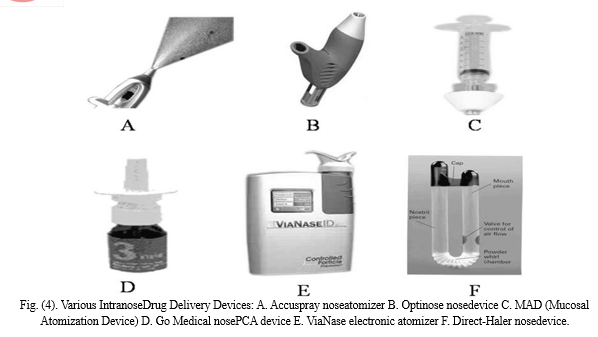
XVII. APPLICATION
Nosedelivery finds sound application to delivery therapeutics to the brain for the management of various brain and brain associated disorders.
A. Neuropathic Pain, Trigeminal Neuralgia And Migraine
Neuropathic pain is a pain caused by damage to or dysfunction of the nervous system. While as, trigeminal neuralgia is a neuropathic pain of one or both of the trigeminal nerves. However, migraine is a neurological syndrome characterized by altered bodily perceptions, severe headaches, and nausea. Wolfe T have reviewed the use of intranoseroute for the management of acute pain using opiates such as fentanyl and sufentanil [215]. In a human study by Huge et al., it was observed that intranoselow dose ketamine rapidly induces adequate plasma concentrations of both ketamine and its metabolite norketamine although intranoseketamine had no significant impact on thermal or mechanical detection and pain thresholds. Kendall et al. compared the effectiveness of intranosediamorphine spray with intramuscular morphine in young people with clinical fractures [216]. Adequate pain relief was achieved by 20 minutes in 95% of patients, irrespective of the method used. However, they observed that the pain relief was achieved quicker with the intranoseadministration than with the intramuscular morphine. Also, the spray did not cause discomfort in most
patients, whereas, most found the intramuscular injection uncomfortable. The US patent 4241301 of Frey et al. describes intranoseadministration of various analgesics like selected from the group consisting of an oxytocin peptide, an enkephalin, an endorphin, a dynorphin, a CGRP antagonist, a CGRP antibody, and an analogue of any of these, for the treatment or prevention of trigeminal neuralgia [217]. Rapoport et al. have thoroughly reviewed the intranoseformulations containing therapeutics like dihydroergotamine mesylate [dihydroergotamine mesilate], sumatriptan, zolmitriptan, butorphanol, capsaicin and lidocaine [lignocaine]] and civamide (a cis-isomer of capsaicin) for the treatment of migraine and cluster headache [218]. Wang et al. have evaluated the efficacy of intranosesumatriptan in the acute treatment of migraine in Taiwanese patients and found a significant difference in headache relief rates between the intranosesumatriptan and placebo treated group [219]. Misra et al. have developed microemulsions containing zolmitriptan and sumatriptan for the management of migraine [34, 164]. Bajaj et al. have reported an intranoseeucalyptus oil containing microemulsion for aromatherapy of migraine [220]. A thermoreversible gel system of sumatriptan has also been develope by Murthy et al. for the management of migraine [165].
XVIII. NEUROAIDS
NeuroAIDS includes neurologic disorders which are a primary consequence of damage to the central and peripheral nervous system by human immunodeficiency virus (HIV) myelopathy, HIV dementia, and cognitive/motor disorder. These syndromes affect 30 to 40% of adults and children with AIDS. Noseroute have been successfully tried for the delivery of antiretrovirals to the brain for the treatment of neuroAIDS. Frey et al. have thoroughly reviewed the various strategies that can be used to deliver antiretrovirals and other drugs to the brain across the nosemucosa for the management oneuroAIDS [221]. The molecules suggested include immunomodulatory and anti-inflammatory agents such as interferon beta-1b and GSK-3beta (upregulated by HIV-1 neurotoxins) inhibitors and neuroprotectives like nerve growth factor (NGF), insulin-like growth factor-I (IGF-I), brainderived neurotrophic factor (BDNF), and EPO. They have reported that anti-inflammatory interferon beta-1b have been successfully delivered to the brain and spinal cord, as well as lymphatics in rodents and in cynomolgus monkeys following noseadministration. Pert et al. have thoroughly reviewed the effects of D-Ala-Peptide T-Amide (DAPTA), A Viral Entry Inhibitor, in humans having neuroAIDS and in humans having other brain disorders [222]. They found intranoseDAPTA to be significantly effective than the conventional approaches in reducing viral load in the brain.
XIX. BRAIN TUMORS
A brain tumor is an abnormal growth of cells within the brain which can be cancerous
(malignant) or non-cancerous (benign). Intranosedelivery of chemotherapeutics to target the CNS has shown great promise for the treatment of brain tumors in preclinical studies. In a study on rats, Tomotaka et al. have reported a significant reduction in the tumor weight with noseapplications of methotrexate compared to the nontreated group and the animals receiving intraperitoneal methotrexate [223]. In another study, Tomotaka et al. Have evaluated the effect of intravenous acetazolamide, an inhibitor of the secretion of CSF, on the transport of 5-fluorouracil following intranoseand intravenous administrations [224]. They found that intravenous acetazolamide markedly increased the concentration of 5-fluorouracil in the CSF and brain following the noseadministration, although the plasma concentrations of the drug were similar with intravenous 5- fluorouracil. The patent WO 2007127163 20071108 describes an invention directed to treat brain tumors by intranasal administration of a telomerase inhibitor in an aerosol composition and also describing the delivery device to be used in the method [225]. The other chemotherapeutic agents studied transnasally include raltitrexed, cisplatin [223, 226].
XX. NEURODEGENERATIVE DISORDERS
Various researchers have tried the novel intranoseroute for the management of neurodegenerative diseases such as Alzheimer’s, dementia, Parkinsonism and cerebral ischemia or stoke. Neurodegeneration is the progressive loss of structure or function of neurons, including death of neurons resulting in neurodegenerative diseases like Parkinson’s, Alzheimer’s, or Huntington’s disease. Frey et al. have studied the uptake of nerve growth factor following intranoseadministrations [227].
Microemulsion based systems of tacrine developed by Misra et al. have demonstrated improvement in memory in scopolamineinduced amnesic mice [160]. Misra et al. have also developed intranosenanoemulsion based formulations of risperidone for the management of Alzheimer’s [228]. Recently, Marshall et al. have demonstrated an enhancement in memory of healthy young volunteers taking interlukin-6 nosespray [229]. Wei et al. have demonstrated that intranoserecombinant human erythropoietin protects rats against focal cerebral ischemia [230]. Wang et al. have reported that intranasally delivered bFGF enhances neurogenesis in adult rats following cerebral ischemia [231].
XXI. EPILEPSY
Intranosedrug delivery route have shown to be promising for the treatment of acute and chronic epilepsy. Wermeling, thoroughly reviews the various benzodiazepines that can be used intranasally for the treatment of epilepsy, including formulation and device considerations, their pharmacology and pharmacokinetic/pharmacodynamic profiles [232]. Deonna et al. evaluated the efficacy, tolerance and applicability of nosemidazolam during acute seizures in children both in hospital and at home [233]. They found nosemidazolam to be effective in the treatment of acute seizures with no serious adverse effects. They also postulated that nosemidazolam was safe to use outside the hospital in severe epilepsies, particularly in older children because it was easy for the parentsto use. Haan et al. compared a novel midazolam HCl concentratednosespray with diazepam rectal solution in the treatment of prolonged seizures in humans [234]. They found that midazolam HCl nosespray was equal to diazepam rectal solution with respect to efficacy and side effects in the suppression of seizure exacerbations. However, majority of the patients and caregivers preferred the nosespray over therectal formulation. Misra et al. found more rapid and larger extent of transport of clonazepam into the rat brain with intranosemucoadhesive microemulsions of clonazepam for the management of epilepsy [161].
A. Sedation/Insomnia/Anxiolysis
Intranoseroute have demonstrated encouraging results with the transport of benzodiazepines to the brain for the management of sleep and anxiety disorders. Misra et al. Have reported microemulsion based intranoseformulations of diazepam, lorazepam and alprazolam for the treatment of insomnia [235]. They observed that the onset of sleep and duration of sleep in male albino rats were in the order: Lorazepam > Alprazolam>Diazepam. In a clinical study on 96 children, Yuen et al. found that intranosedexmedetomidine produced more sedation than oral midazolam, but with similar and acceptable cooperation. In invention WO/2006/071274, Gregory et al. have described an intranosecomposition of a NMDA receptor antagonist and an acetylcholinesterase inhibitor for the treatment of alzheimer’s disease.
XXII. DELIVERY OF PROTEIN THERAPEUTICS TO THE BRAIN
Due to an extensive research in the field of protein based therapeutics such as therapeutic peptides, proteins, and vaccines, intranosedelivery depicts an attractive administration route. oral administration results in poor bioavailability of these molecules due to their large size and rapid enzymatic degradation [237]. Hence, these therapeutics are generally administered intravenously to avoid physicochemical instability and hepatic first pass metabolism. Intranoseadministrationseems to be promising option as demonstrates minimal enzymatic degradation of protein therapeutics and avoids first pass metabolism. It can be used to deliver drugs both to the systemic circulation and to the brain. Although, several intranoseformulations are marketed for systemic drug delivery, only desmopressin and oxytocin noseformulations are marketed for drug delivery to the brain [238]. Several other protein based noseformulations are under clinical investigations. Delivery of protein therapeutics to the brain majorly involves extraneuronal transport as it occurs within minutes. A number of protein therapeutics have been successfully delivered to the brain using intranoseroute such as NGF [227, 239, 240], IGF-I [241], FGF [242] and ADNF [243] in animals. In humans, proteins such as AVP [244], CCK analog [245], MSH/ACTH [246, 247] and insulin [248, 249] have been successfully transported to the brain following noseadministration. Recently, the U.S. Centers for Disease Control and Prevention have disclosed that the first U.S. H1N1 vaccines against swine flu will be in the form of nosesprays. Hussain, has reviewed the various animal models to study the noseabsorption and the effect of physicochemical and biopharmaceutical properties on the rate and extent of drug absorption [250]. He has also discussed the factors affecting peptide absorption and methods to improve the noseabsorption of peptides. Frey et al. Have studied the brain uptake of peptoid CHIR5585, an antagonist of the urokinase plasminogen activator receptor (uPAR),n following intranoseadministration [251]. Peptoids are a novel class of peptide isomers that are oligomeric Nsubstituted glycine peptides. Intranoseadministrations resulted in significant delivery of the protein throughout the CNS with autoradiography demonstrating a similar distribution pattern.
Frey et al. have also studied the transnosedelivery of cells to the brain following intranoseapplication of fluorescently labeled rat mesenchymal stem cells (MSC) or human glioma cells to naive mice and rats [252]. Recently, Jamie T. has reported the delivery of stem cells to the brain through the noseroute [253].
XXIII. DELIVERY OF DNA PLASMIDS TO THE BRAIN
The several routes available for mucosal immunization, the noseroute is particularly attractive because of the ease of administration and the induction of potent immune responses even at distal mucosal sites. However, adjuvants are required to enhance the immune responses after noseimmunization. The use of nosepoly(lactide co-glycolide) microparticles as adjuvants and delivery systems for protein and DNA vaccines for mucosal immunization have been reviewed by Vajdy et al. [254]. They reported that following intranoseadministration of DNA plasmids, the level of plasmids in the brain were 3.9 to 4.8 times higher than the plasmid concentration in the lungs and spleen. It was also found that the plasmid DNA reached the brain within 15 min following noseinstillation [255]. The higher distribution of plasmid to the brain following intranoseadministration indicates that noseroute might serve as a potential route for the delivery of therapeutic genes to the brain with minimal sideeffects in non-target organs. However, the efficacy of the proteinaceous vaccines following noseadministration is dependent on the molecular structure and size of the protein. Although, several intranosevaccines are marketed against upper respiratory tract infections like influenza none is available for CNS application [256].
XXIV. OTHERS
Intranoseroute have also been reported in the treatment of brain and brain associated disorders like obesity, erectile dysfunction, smoking cessation, multiple sclerosis, restless leg syndrome, etc. In a clinical investigation on humans, Greenway et al. determined the effect of intranoselidocaine on food intake and found that although intranoselidocaine reduced hunger and the desire to eat, did not demonstrated significant reduction in food intake suggesting that intranoselidocaine will not have value in treating obesity [257]. Misra et al. have demonstrated the potential of intranosecabergoline, a D2 receptor agonist, in the management of obesity in mice [258]. They postulate that long-term studies in at least two more animal models followed by extensive clinical evaluation should be carried out for a clinically acceptable product. Hallschmid et al. have demonstrated that intranoseinsulin reduces body fat in men but not in women [259]. Nicotine nosespray is being marketed for the management of smoking cessation [238]. European Patent EP1547592 describes an intranoseaqueous solution of rotigotine for the treatment of morbus Parkinson and restless leg syndrome [260]. Frey et al. have demonstrated greater CNS uptake of interferon beta following intranoseadministration to be used for the treatment of multiple sclerosis [261]. European Patent EP0967214 by Billotte et al. describes intranoseformulations of cGMP PDE5 enzyme inhibitors like sildenafil mesylate for the treatment of male erectile dysfunction [262].
XXV. INVESTIGATIONAL AND MARKETED PRODUCTS
Intranoseadministration is a rapid and safe method not only for the patient but also for the provider to combat emergency situations. However, due to aggressive research in the field of nosedrug delivery, numerous conspicuous merits and features have attracted pharmaceutical industries to design the products as intranosedelivery systems. A few of the marketed and investigational nose to brain targeted productsn are listed in Table 2 and 3 respectively [22, 238, 263].
XXVI. FUTURE STRATEGIES
Several novel approaches have been researched to enhance drug absorption across nosemucosa and are discussed in this review. Still, few unconventional strategies can be explored for delivering drugs effectively across the nosemucosa. Various strategies have been described by different authors to enhance the uptake of therapeutics transnasally [26, 162, 252].
Conclusion
The nose to brain drug delivery has proven its worth by the presence of many commercially successful products. Promising results have been observed even with large molecules such as peptides, proteins, hormones and stem cells. These reported findings may be crucial in developing therapeutically efficacious product in the management of chronic brain diseases otherwise difficult to treat such as brain tumors, epilepsy, migraine and neurodegenerative diseases. Few researchers have reported utility of intranoseroute in humans to treat brain diseases with minimal unwanted systemic drug disposition compared to other routes of drug administration including oral or parenteral. Despite the enormous progress, still there is a need for a device for selective delivery of the product at the olfactory region in the nosecavity. Methods should be investigated to deliver drugs tothe specific brain areas affected in the respective neurological disorder such as the brainstem and cerebellum in Parkinson’s disease while, the frontal cortex in Alzheimer’s disease,dementia, or personality disorders. To conclude, the future of nose to brain drug delivery lies in the development of novel formulations that selectively and effectively deliver drugs to specific brain areas without any significant local or systemic toxicities. A. Conflict Of Interest The author(s) confirm that this article content has no conflicts of interest. B. Acknowledgement Declared none.
References
[1] Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx., 2005, 2, 3-14. [2] Rapoport, S.I. Blood brain barrier in physiology and medicine; Raven Press: New York, 1976. [3] Butte, A.M.; Jones, H.C.; Abbot, N.J. Electrical resistance across the blood-brain barrier in anaesthetized rats; a development study. J. Physiol., 1990, 429, 47-62. [4] Crone, C.; Suckling, A.J.; Rumsby, M.G.; Bradbury, M.W.B. The Blood–Brain Barrier in Health and Disease; Ellis Harwood:Chichester, 1986, pp. 17-40. [5] Brightman, M. Handbook of experimental pharmacology; Springer-Verlag: Berlin, 1992, pp. 1-22. [6] Lo, E.H.; Singhal, A.B.; Torchilin, V.P.; Abbott, N.J. Drug delivery to damaged brain. Brain Res. Rev., 2001, 38(1), 140-148. [7] Loscher, W.; Potschka, H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol., 2005, 76(1), 22-76. [8] Misra, A.; Ganesh, S.; Shahiwala, A.; Shah, S.P. Drug delivery to the central nervous system: a review. J. Pharm. Pharmaceut. Sci., 2003, 6(2), 252-273. [9] Smith, Q.R. Advances in Neurology. In: Alzheimer’s Diseases, Wurtman R, Eds.; Raven Press: New York, 1990, Vol. 51, pp.217- 22. [10] Talegaonkar, S.; Mishra, P.R. Intranosedelivery: An approach to bypass the blood brain barrier. Indian. J. Pharmacol., 2004, 36(3),40-147. [11] Begley, D.J.; Bradbury, M.W.; Kreuter, J. The blood brain barrier and drug delivery to the CNS; Marcel Dekker: New York, 1998, pp. 122. [12] Report linker: Find industry reports, company profiles and market statistics. http://www.reportlinker.com/p0164267/Intranasal-Drug-Delivery-US-Market-Trends-andAnalysis.html (Accessed March 8, 2010). [13] Illum, L. Nosedrug delivery-possibilities, problems and solutions. J. Control. Release, 2003, 87(1-3), 187-198. [14] Van den Berg, M.P.; Merkus, P.; Romeijn, S.G.; Verhoef, J.C., Merkus, F.W. Hydroxocobalamin Uptake into the Cerebrospinal Fluid after Noseand Intravenous Delivery in Rats and Humans. J. Drug Target., 2003, 11(6), 325-31. [15] Dahlin, M.; Bjork, E. Noseadministration of a physostigmine analogue (NXX-066) for Alzheimer\'s disease to rats. Int. J. Pharm., 2001, 212(2), 267-274. [16] Bagger, M.A.; Bechgaard, E. The potential of noseapplication for delivery to the central brain-a microdialysis study of fluorescein in rats. Eur. J. Pharm. Sci., 2004, 21(2-3), 235-42. [17] Balmer, C.W.; LaMantia, A.S. Noses and neurons: induction, morphogenesis, and neuronal differentiation in the peripheral olfactory pathway. Dev. Dyn., 2005, 234(3), 464481. [18] Jones, N. The nose and paranosesinuses physiology and anatomy. Adv. Drug Deliv. Rev., 2001, 51(1-3), 5-19. [19] Van Cauwenberge, P.; Sys, L.; De Belder, T.; Watelet, J.B. Anatomy and physiology of the nose and the paranosesinuses. Immunol. Allergy Clin. North Am., 2004, 24(1), 1-17. [20] Watelet, J. B.; Van Cauwenberge, P. Applied anatomy and physiology of the nose and paranosesinuses. Allergy, 1999 54(57), 14- 25. [21] Pires, A.; Fortuna, A.; Alves, G.; Falcao, A. IntranoseDrug Delivery: How, Why and What for? J. Pharm. Pharmaceut. Sci., 2009, 12(3), 288-311. [22] Vyas, T.K.; Shahiwala, A,; Sudhanva Marathe, S.; Misra, A. IntranoseDrug Delivery for Brain Targeting. Curr. Drug Deliv., 2005, 2(2), 165-175. [23] Mygind, N.; Anggard, A. Anatomy and physiology of the nosepathophysiologic alterations in allergic rhinitis. Clin. Rev. Allergy, 1984, 2(3), 173-188. [24] Arora, P.; Sharma, S.; Garg, S. Permeability issues in nosedrug delivery. Drug Discov. Today, 2002, 7(18), 967-975. [25] Brandtzaeg, P. Role of secretory antibodies in the defence against infections. Int. J. Med. Microbiol., 2003, 293(1), 3-15. [26] Dhuria, S.V., Hanson, L.R., Frey II, W.H. IntranoseDelivery to the Central Nervous System: Mechanisms and Experimental Considerations. J. Pharmaceut. Sci., 2010, 99(4), 1654-1673. [27] Kaliner, M.;, Shelhamer, J.; Borson, B.; Nadel, J.; Patow, C.; Marom, Z.;. Human respiratory mucus. Am. Rev. Respir. Dis., 1986, 134(3), 612-621. [28] Ding, X.; Kaminsky, L.S. Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu. Rev. Pharmacol. Toxicol., 2003, 43, 149-173. [29] Minn, A.; Leclerc, S.; Heydel, J.M.; Minn, A.L.; Denizcot, C.; Cattarelli, M.; Netter, P.; Gradinaru, D. Drug transport into the mammalian brain: the nosepathway and its specific metabolic barrier. J Drug Targe.t, 2002, 10(4), 285-296. [30] Agarwal, V.; Mishra, B. Recent trends in drug delivery systems: intranosedrug delivery. Indian J. Exp. Biol., 1999, 37(1), 6-16. [31] Aurora, J. Development of nosedelivery systems: a review. Drug Deliv. Tech., 2002, 2(7), 1-8. [32] Jones, N.S.; Quraishi, S.; Mason, J.D. The nosedelivery of systemic drugs. Int. J. Clin. Pract., 1997, 51(5), 308-311. [33] Wermeling, D.P.; Miller, J.L.; Rudy, A.C. Systematic intranosedrug delivery: concepts and applications. Drug Deliv. Tech., 2002, 2(1), 56-61. [34] Vyas, T.K.; Babbar, A.K.; Sharma, R.K.; Misra, A. Intranosemucoadhesive microemulsions of zolmitriptan: preliminary studies on brain-targeting. J. Drug Target., 2005, 13(5), 317-324. [35] Isaka M.; Yasuda, Y.; Mizokami, M.; Kozuka, S.; Taniguchi, T.; Matano, K.; Maeyama, J.; Mizuno, K.; Morokuma, K.; Ohkuma, K.; Goto, N.; Tochikubo, K. Mucosal immunization against hepatitis B virus by intranoseco-administration of recombinant hepatitis B surface antigen and recombinant cholera toxin B subunit as an adjuvant. Vaccine, 2001, 19(11-12), 1460-1466. [36] Romeo, V.D.; De Meireles, J.; Sileno, A.P.; Pimplaskar, H.K.; Behl, C.R. Effects of physiochemical properties and other factors on systemic nosedrug delivery. Adv. Drug Del. Rev., 1998, 29, 89- 116. [37] Fisher, A.N.; Brown, K.; Davis, S.S.; Parr, G.D.; Smith, D.A. J. Pharm. Pharmacol., 1987, 39, 357-362. [38] Jones, N.S.; Quraishi, S.; Mason, J.D.T. The nosedelivery of systemic drugs. Int. J. Clin. Pract., 1997, 51 (5), 308-311. [39] Ying, W. The nose may help the brain: intranosedrug delivery for treating neurological diseases. Future Neurol., 2008, 3(1), 1-4. [40] McMartin, C.; Hutchinson, L.E.F.; Hyde, R.; Peters, G.E. Analysis of structural requirements for the absorption of drugs and macromoleculesfrom the nosecavity. J. Pharm. Sci., 1987, 76(7), 535 540. [41] Gavini, E.; Rassu, G.; Sanna, V.; Cossu, M.; Giunchedi, P. Mucoadhesive microspheres for noseadministration of an antiemetic drug, metoclopramide: in-vitro/ex-vivo studies. J. Pharm. Pharmacol., 2005, 57(3), 287-294. [42] IIlum, L. Nosedrug delivery: new developments and strategies. Drug Discov. Today, 2002, 7(23), 1184-1189. [43] IIlum, L. Transport of drugs from the nosecavity to the central nervous system. Eur. J. Pharm. Sci., 2000, 11, 1-18. [44] Jansson, B., Bjork, E. Visualization of in vivo olfactory uptake and transfer using fluorescein dextran. J. Drug Target., 2002, 10, 379-386. [45] Banks, W.A.; During, M.J.; Niehoff, M.L. Brain uptake of theglucagon-like peptide-1 antagonist exendin(9-39) after intranoseadministration. J. Pharmacol. Exp. Ther., 2004, 309, 469-475. [46] Ross, T.M.; Martinez, P.M.; Renner, J.C.; Thorne, R.G.; Hanson, L.R.; Frey, W.H.II. Intranoseadministration of interferon beta bypasses the blood brain barrier to target the central nervous system and cervical lymph nodes: A non-invasive treatment strategy for multiple sclerosis. J. Neuroimmunol., 2004, 151, 66-77. [47] Thorne, R.G.; Hanson, L.R.; Ross, T.M.; Tung, D.; Frey, W.H.II. Delivery of interferonb to the monkey nervous system following intranoseadministration. Neuroscience, 2008, 152(3), 785-797. [48] Buck, L.B. The chemical senses. In: Principles of neural science, 4th ed; Kandel, E.R., Schwartz, J.H., Jessell, T.M., Eds; McGraw- Hill Companies: New York, 2000, pp. 625-652. [49] Clerico, D.M., To, W.C., Lanza, D.C. Anatomy of the human nosepassages. In: Handbook of olfaction and gestation, 2nd ed; Doty, R.L., Eds; Marcel Dekker Inc.: New York, 2003, pp. 1-16. [50] Gray, H. Gray’s anatomy, 15th ed (Classic Collectors edition). Bounty Books: New York, 1978. [51] Schaefer, M.L.; Bottger, B.; Silver, W.L.; Finger, T.E. Trigeminal collaterals in the noseepithelium and olfactory bulb: A potential route for direct modulation of olfactory information by trigeminal stimuli. J. Comp. Neurol., 2002, 444(3), 221-226. [52] Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H.II. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranoseadministration. Neuroscience, 2004, 127(2), 481-496. [53] Cauna, N. Blood and nerve supply of the noselining. In: The nose: Upper airway physiology and the atmospheric environment, Proctor, D.F., Andersen, I., Eds; Elsevier Biomedical Press: Amsterdam, 1982, pp. 45-69. [54] DeSesso, J.M. The relevance to humans of animal models for inhalation studies of cancer in the nose and upper airways. Qual. Assur., 1993, 2(3), 213-231. [55] Grevers, G.; Herrmann, U. Fenestrated endothelia in vessels of the nosemucosa. An electron-microscopic study in the rabbit. Arch. Otorhinolaryngol., 1987, 244(1), 55-60. [56] Van Diest, P.; Kanan, M.W. An ultrastructural study of the endonosemicrocirculation in the Wistar rat during fetal and early postnatal life. J. Anat., 1979, 128(Pt 2), 293-300. [57] Einer-Jensen, N.; Hunter, R. Counter-current transfer in reproductive biology. Reproduction, 2005, 129(1), 9-18. [58] Einer-Jensen, N.; Larsen, L. Local transfer of diazepam, but not of cocaine, from the nosecavities to the brain arteria blood in rats. Pharmacol. Toxicol., 2000, 87(6), 276-278. [59] Skipor, J.; Grzegorzewski, W.; Einer-Jensen, N.; Wasowska, B. Local vascular pathway for progesterone transfer to the brain after noseadministration in gilts. Reprod. Biol., 2003, 3(2), 143-159. [60] Pollock, H.; Hutchings, M.; Weller, R.O.; Zhang, E.T. Perivascular spaces in the basal ganglia of the human brain: Their relationship to lacunes. J. Anat., 1997, 191(Pt 3), 337-346. [61] Bradbury, M.W.; Cserr, H.F.; Westrop, R.J. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am. J. Physiol., 1981, 240(4), F329–F336. [62] Carare, R.O.; Bernardes-Silva, M.; Newman, T.A.; Page, A.M.; Nicoll, J.A.; Perry, V.H.; Weller, R.O. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol., 2008, 34(2), 131-144. [63] Li, Y.X.; Chen, L.B.; Xia, Z.L.; Yang, M.F.; Zhang, Y.Z.; Zhang, X.Y. Drainage of macromolecules from the Caudato-Putamen of rat brain. Chin. J. Physiol., 2005, 48(1), 7-14. [64] Zhang, E.T.; Richards, H.K.; Kida, S.; Weller, R.O. Directional and compartmentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta Neuropathol., 1992, 83(3), 233-239. [65] Cserr, H.F.; Cooper, D.N.; Suri, P.K.; Patlak, C.S. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol., 1981, 240(4), F319-F328. [66] Groothuis, D.R.; Vavra, M.W.; Schlageter, K.E.; Kang, E.W.; Itskovich, A.C.; Hertzler, S.; Allen, C.V.; Lipton, H.L. Efflux of drugs and solutes from brain: The interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters. J. Cereb. Blood Flow Metab., 2007, 27(1), 43-56. [67] Rennels, M.L.; Blaumanis, O.R.; Grady, P.A. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv. Neurol., 1990, 52, 431-439. [68] Rennels, M.L.; Gregory, T.F.; Blaumanis, O.R.; Fujimoto, K.; Grady, P.A. Evidence for a ’paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distributionof tracer protein throughout the brain from the subarachnoid space. Brain Res., 1985, 326(1), 47-63. [69] Dhuria, S.V.; Hanson, L.R.; Frey, W.H.II. Novel vasoconstrictor formulation to enhance intranosetargeting of neuropeptide therapeutics to the central nervous system. J. Pharmacol. Exp. Ther., 2009, 328(1), 312-320. [70] Bradbury, M.W.; Westrop, R.J. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J. Physiol., 1983, 339, 519-534. [71] Johnston, M.; Zakharov, A.; Papaiconomou, C.; Salmasi, G.; Armstrong, D. Evidence of connections between cerebrospinal fluid and noselymphatic vessels in humans, non-human primates and other species. Cerebrospinal Fluid Res., 2004, 1(1), 2. [72] Kida, S.; Pantazis, A.; Weller, R.O. CSF drains directly from the subarachnoid space into noselymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol., 1993, 19(6), 480-488. [73] Walter, B.A.; Valera, V.A.; Takahashi, S.; Ushiki, T. The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol. Appl. Neurobiol., 2006, 32(4), 388-396. [74] Dhanda, D.S.; Frey, W.H.II.; Leopold, D.; Kompella, U.B. Approaches for drug deposition in the human olfactory epithelium. Drug Deliv. Tech., 2005, 5, 64-72. [75] Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing neuropeptides: A transnoseapproach to the human brain. Nat. Neurosci., 2002, 5, 514-516. [76] Sakane, T.; Akizuki, M.; Yamashita, S.; Sezaki, H.; Nadai, T. Direct drug transport from the rat nosecavity to the cerebrospinal fluid: The relation to the dissociation of the drug. J. Pharm. Pharmacol., , 46(5), 378-379. [77] Wang, Q.; Chen, G.; Zeng, S. Pharmacokinetics of Gastrodin in rat plasma and CSF after i.n. and i.v. Int. J. Pharm., 2007, 341(1-2), 20-25. [78] Boulton, M.; Armstrong, D.; Flessner, M.; Hay, J.; Szalai, J.P.; Johnston, M. Raised intracranial pressure increases CSF drainage through arachnoid villi and extracranial lymphatics. Am. J. Physiol., 1998, 275(3 Pt 2), R889-R896. [79] Boulton, M.; Flessner, M.; Armstrong, D.; Mohamed, R.; Hay, J.; Johnston, M. Contribution of extracranial lymphatics and arachnoid villi to the clearance of a CSF tracer in the rat. Am. J. Physiol., 1999, 276(3 Pt 2), R818-R823. [80] Johnston, M.; Zakharov, A.; Koh, L.; Armstrong, D. Subarachnoid injection of microfil reveals connections between cerebrospinalfluid and noselymphatics in the non-human primate. Neuropathol. Appl. Neurobiol., 2005, 31(6), 632-640. [81] Papaiconomou, C.; Bozanovic-Sosic, R.; Zakharov, A.; Johnston, . Does neonatal cerebrospinal fluid absorption occur via arachnoid projections or extracranial lymphatics? Am. J. Physiol. Regul. Integr. Comp. Physiol., 2002, 283(4), R869-R876. [82] Filippidis, A.; Fountas, K.N. Noselymphatics as a novel invasion and dissemination route of bacterial meningitis. Med. Hypotheses, 2009, 72(6), 694-697. [83] Vidgren, M.T.; Kublik, H. Nosedelivery systems and their effect on deposition and absorption. Adv. Drug Deliv. Rev., 1998, 29(1-2), 157-177. [84] Turker, S.; Onur, E.; Ozer, Y. Noseroute and drug delivery systems. Pharm. World Sci., 2004, 26(3), 137-142. [85] Michael, I.; Ugwoke, M.I.; Agu, R.U.; Verbeke, N.; Kinget, R. Nosemucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev., 2005, 57(11), 1640-1665. [86] Dahl, A.R.; Lewis, J.L. Respiratory tract uptake of inhalants and metabolism of xenobiotics. Annu. Rev. Pharmacol. Toxicol., 1993, 33, 383-407. [87] Sarkar, M.A. Drug metabolism in the nosemucosa. Pharm. Res., 1992, 9(1), 1-9. [88] Harris, A.S.; Nilsson, I.M.; Wagner, Z.G.; Alkner, U. Intranoseadministration of peptides: nosedeposition, biological response and absorption of desmopressin. J. Pharm. Sci., 1986, 75(11), 1085-1088. [89] Machida, M.; Sano, K.; Arakawa, M.; Hayashi, M.; Awazu, S. Effects of surfactants and protease inhibitors on noseabsorption of recombinant human granulocyte colonystimulating factor (rHGCSF) in rats. Biol. Pharm. Bull., 1994, 17(10), 1375-1378. [90] Bernkop-Schnurch, A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J. Control. Release, 1998, 52(1-2), 1-16. [91] Raehs, S.C.; Sandow, J.; Wirth, K.; Merkle, H.P. The adjuvant effect of bacitracin on noseabsorption of gonadorelin and buserelin in rats. Pharm. Res., 1988, 5(11), 689-693. [92] Hussain, M.A.; Shenvi, A.B.; Rowe, S.M.; Shefter, E. The use of alpha-aminoboronic acid derivatives to stabilize peptide drugs during their intranoseabsorption. Pharm. Res., 1989, 6(2), 186-189. [93] O\'Hagan, D.T.; Critchley, H.; Farraj, N.F.; Fisher, A.N.; Johansen, B.R.; Davis, S.S.; Illum, L. Noseabsorption enhancers for biosynthetic human growth hormone in rats. Pharm. Res., 1990, 7(7), 772-776. [94] Huang, C.H.; Kimura, R.; Nassar, R.B.; Hussain, A. Mechanism of noseabsorption of drugs I: Physicochemical parameters influencing the rate of in situ noseabsorption of drugs in rats. J. Pharm. Sci., 1985, 74(6), 608-611. [95] Krishnamoorthy, R.; Mitra, A.K. Prodrugs for nosedrug delivery. Adv. Drug Deliv. Rev., 1998, 29(1-2), 135-146. [96] Yang, P. The effect of nosesensory nerve on the enhanced noseblood vessel permeability caused by histamine. Zhonghua Er Bi Yan Hou Ke Za Zhi, 1991, 26(4), 207-208. [97] Philip, G.; Baroody, F.M.; Proud, D.; Naclerio, R.M.; Togias, A.G. The human noseresponse to capsaicin. J. Allergy Clin. Immunol., 1994, 94(6 Pt 1), 1035-1045. [98] Tos, M. Distribution of mucus producing elements in respiratory tract. Differences between upper and lower airways. Eur. J. Respir. Dis., 1983, 64(Suppl. 128), 269-279. [99] Misawa, M. A new rhinitis model using chemical mediators in rats. Jpn. J. Pharmacol., 1988, 48(1), 15-22. [100] Newman, S.P.; Moren, F.; Clarke, S.W. Deposition pattern from a nosepump spray. Rhinology, 1987, 25(2), 77-82. [101] Newman, S.P.; Moren, F.; Clarke, S.W. Deposition pattern of nosesprays in man. Rhinology, 1988, 26(2), 111-120. [102] Dalton, M.E.; Bromham, D.R.; Ambrose, C.L.; Osborne, J.; Dalton, K.D. Noseabsorption of progesterone in women. Br. J. Obstet. Gynaecol., 1987, 94(1), 84-88. [103] Costantino, H.R.; Illum, L.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranosedelivery: physicochemical and therapeutic aspects. Int. J. Pharm., 2007, 337(1-2), 1-24. [104] Graff, C.L.; Pollack, G.M. Functional Evidence for P-glycoprotein at the Nose-Brain Barrier. Pharm. Res., 2005, 22(1), 86-93. [105] Graff, C.L.; Pollack, G.M. P-Glycoprotein attenuates brain uptake of substrates after noseinstillation. Pharm. Res., 2003, 20(8), 1225-1230. [106] Westin, U.; Piras, E.; Jansson, B.; Bergstrom, U.; Dahlin, M.; Brittebo, E.; Bjork, E. Transfer of morphine along the olfactory pathway to the central nervous system after noseadministration to rodents. Eur. J. Pharm. Sci., 2005, 24(5), 565-573. [107] Kao, H.D.; Traboulsi, A.; Itoh, S.; Dittert, L.; Hussain, A. Enhancement of the systemic and CNS specific delivery of L-dopa by the noseadministration of its water soluble prodrugs. Pharm. Res., 2000, 17(8), 978-984. [108] Mortazavi, S.A.; Smart, J.D. Factors influencing gel strengthening at the mucoadhesive-mucus interface. J. Pharm. Pharmacol., 1994, 46(2), 86-90. [109] Merkus, F.W.; Verhoef, J.C.; Schipper, N.G.; Marttin, E. Nosemucociliary clearance as a factor in nosedrug delivery. Adv. Drug Deliv. Rev., 1998, 29(1-2), 13-38. [110] Schipper, N.G.M.; Verhoef, J.C.; Merkus, F.W.H.M. The nosemucociliary clearance: relevance to nosedrug delivery. Pharm. Res., 1991, 8(7), 807-814. [111] Cornaz, A.L.; Buri, P. Nosemucosa as an absorption barrier. Eur. J. Pharm. Biopharm., 1994, 40, 261-270. [112] Tafaghodi, M.; Tabassi, S.A.S.; Jaafari, M.R.; Zakavi, S.R.; Momen- Nejad, M. Evaluation of the clearance characteristics of various microspheres in the human nose by gamma-scintigraphy. Int. J. Pharm., 2004, 280(1-2), 125-135. [113] Gizurarson, S. The relevance of nosephysiology to the design of drug absorption studies. Adv. Drug Del. Rev., 1993, 11(3), 329- 347. [114] Merkus P, Romeijn SG, Verhoef JC, Merkus FW, Schouwenburg PF. Classification of cilio-inhibiting effects of nosedrugs. Laryngoscope, 2001, 111(4 Pt 1), 595-602. [115] Corbo, D.C. Characterization of the barrier properties of mucosal membranes. J. Pharm. Sci., 1990, 79(3), 202-206. [116] Corbo, D.C. Drug absorption through mucosal membranes: effect of mucosal route and penetrant hydrophilicity. Pharm. Res., 1989, 6(10), 848-852. [117] Huang, C.H.; Kimura, R.; Bawarshi-Nassar, R.; Hussain, A. Mechanism of noseabsorption of drugs. II: Absorption of Ltyrosine and the effect of structural modification on its absorption. J. Pharm. Sci., 1985, 74(12), 1298-1301. [118] Doelker, E. Crystalline modifications and polymorphism changes during drug manufacture. Ann. Pharm. Fr., 2002, 60(3), 161-176. [119] Morissette, S.L.; Almarsson, O.; Peterson, M.L.; Remenar, J.F.; Read, M.J.; Lemmo, A.V.; Ellis, S.; Cima, M.J.; Gardner, C.R. High-throughput crystallization: polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv. Drug Deliv. Rev., 2004, 56(3), 275300. [120] Raw, A.S.; Furness, M.S.; Gill, D.S.; Adams, R.C.; Holcombe Jr., F.O., Yu, L.X. Regulatory considerations of pharmaceutical solid polymorphism in Abbreviated New Drug Applications (ANDAs). Adv. Drug Deliv. Rev., 2004, 56(3), 397-414. [121] Matsuyama, T.; Morita, T.; Horikiri, Y.; Yamahara, H.; Yoshino, H. Enhancement of noseabsorption of large molecular weight compounds by combination of mucolytic agent and nonionic surfactant. J. Control. Release, 2006, 110(2), 347-352. [122] Fisher, A.N.; Illum, L.; Davis, S.S.; Schacht, E.H. Di-iodo-Ltyrosine labelled dextrans as molecular size markers of noseabsorptionin rat. J. Pharm. Pharmacol., 1992, 44(7), 550554. [123] Yamamoto, A.; Iseki, T.; Ochi-Sugiyama, M.; Okada, N.; Fujita, T,; Muranishi, S. Absorption of water-soluble compounds with different molecular weights. J. Control. Release, 2001, 76(3), 363- 374. [124] Jiang, X.G.; Lu, X.; Cui, J.B.; Qiu, L.; Xi, N.Z. Studies on octanolwater partition coefficient and nosedrug absorption. Yao Xue Xue Bao, 1997, 32(6), 458-460. [125] Hirai, S.; Yashiki, T.; Matsuzawa, T.; Mima, H. Absorption of drugs from the nosemucosa of rat. Int. J. Pharm., 1981, 7, 317- 325. [126] McMartin, C.; Hutchinson, L.E.; Hyde, R.; Peters, G.E. Analysis of structural requirements for the absorption of drugs and macromolecules from the nosecavity. J. Pharm. Sci., 1987, 76(7), 535-540. [127] Rathbone, M.J. Nosesystemic drug delivery. N. Z. Pharm., 1994, 14, 37-39. [128] Ohwaki, T.; Ando, H.; Watanabe, S.; Miyake, Y. Effects of dose, pH, and osmolarity on noseabsorption of secretin in rats. J. Pharm. Sci., 1985, 74(5), 550-552. [129] Ohwaki, T.; Ando, H.; Kakimoto, F.; Uesugi, K.; Watanabe, S.; Miyake, Y.; Kayano, M. Effects of dose, pH, and osmolarity on noseabsorption of secretin in rats. II: Histological aspects of the nosemucosa in relation to the absorption variation due to the effects of pH and osmolarity. J. Pharm. Sci., 1987, 76(9), 695-698. [130] Ohwaki, T.; Ishii, M.; Aoki, S.; Tatsuishi, K.; Kayano, M. Effect of dose, pH, and osmolarity on noseabsorption of secretin in rats. III. In vitro membrane permeation test and determination of apparent partition coefficient of secretion. Chem. Pharm. Bull. (Tokyo), 1989, 37(12), 3359-3362. [131] Jansson, B.; Hagerstrom, H.; Fransen, N.; Edsman, K.; Bjork, E. The influence of gellan gum on the transfer of fluorescein dextran across rat noseepithelium in vivo, Eur. J. Pharm. Biopharm., 2005, 59(3), 557-564. [132] Suzuki, Y.; Makino, Y. Mucosal drug delivery using cellulose derivatives as a functional polymer. J. Control. Release, 1999, 62(1-2), 101-107. [133] Hounam, R.F.; Black, A.; Walsh, M. The deposition of aerosol particles in the nasopharyngal region of the human respiratory tract. Aerosol Sci., 1971, 2, 47-61. [134] Bates, D.V.; Fish, B.R.; Hatch, T.F.; Mercer, T.T.; Marrow, P.E. Deposition and retention models for internal dosimetry of human respiratory tract. Task group on lung dyanamics. Health Phys., 1966, 12(2), 173-207. [135] Patel, R.S.; McGarry, G.W. Most patients overdose on topical nosecorticosteroid drops: an accurate delivery device is required. J. Laryngol. Otol., 2001, 115(8), 633-635. [136] Ishikawa, F.; Katsura, M.; Tamai, I.; Tsuji, A. Improved nosebioavailability of elcatonin by insoluble powder formulation. Int. J. Pharm., 2001, 224(1-2), 105-114. [137] Junginger, H.E. Mucoadhesive hydrogels. Pharmazeutische Industrie, 1991, 53(11), 1056-1065. [138] Bagool, M.A. Review article on topical ocular drug delivery. Indian Drugs, 1994, 31(10), 451-456. [139] Mitra, R.; Pezron, I.; Chu, W.A.; Mitra, A.K. Lipid emulsions as vehicles for enhanced nosedelivery of insulin. Int. J. Pharm., 2000, 205(1-2), 127-134. [140] El-Shafy, M.A.; Kellaway, I.W.; Taylor, G.; Dickinson, P.A. Improved nose bioavailability of FITCdextran (MW 4300) from mucoadhesive microspheres in rabbits. J. Drug Target., 2000, 7(5), 355-361. [141] D’Souza, S.A D\'Souza, S.A.; Ray, J.; Pandey, S.; Udupa, N. Absorption of ciprofloxacin and norfloxacin when administered as niosome-encapsulated inclusion complexes. J. Pharm. Pharmacol., 1997, 49(2), 145-149. [142] Lim, S.T.; Forbes, B.; Berry, D.J.; Martin, G.P.; Brown, M.B. In vivo evaluation of novel hyaluronan/chitosan microparticulate delivery systems for the nosedelivery of gentamicin in rabbits. Int. J. Pharm., 2002, 231(1), 73-82. [143] Muramatsu, K.; Maitani, Y.; Takayama, K.; Nagai, T. The relationship between the rigidity of the liposomal membrane and the absorption of insulin after noseadministration of liposomes modified with an enhancer containing insulin in rabbits. Drug Dev. Ind. Pharm., 1999, 25(10), 1099-1105. [144] Pitha, J.; Irie, T.; Sklar, P.B.; Nye, J.S. Drug solubilizers to aid pharmacologists: amorphous cyclodextrin derivatives. Life Sci., 1988, 43(6), 493-502. [145] Shimpi, S.; Chauhan, B.; Shimpi, P. Cyclodextrins: application in different routes of drug administration. Acta Pharm., 2005, 55(2), 139-156. [146] Waterman, K.C.; Adami, R.C.; Alsante, K.M.; Hong, J.; Landis, M.S.; Lombardo, F.; Roberts, C.J. Stabilization of pharmaceuticals to oxidative degradation. Pharm. Dev. Techno.l, 2002, 7(1), 1-32. [147] Ohtake, K.; Maeno, T.; Ueda, H.; Natsume, H.; Morimoto, Y. Poly- L-arginine predominantly increases the paracellular permeability of hydrophilic macromolecules across rabbit noseepithelium in vitro. Pharm. Res., 2003, 20(2), 153-160. [148] Zhang, Y.J.; Ma, C.H.; Lu, W.L.; Zhang, X.; Wang, X.L.; Sun, J.N.; Zhang, Q. Permeation-enhancing effects of chitosan formulations on recombinant hirudin-2 by nosedelivery in vitro and in vivo. Acta Pharmacol. Sin., 2005, 26(11), 1402-1408. [149] Lebe, E.; Baka, M.; Yavasoglu, A.; Aktug, H.; Ates, U.; Uyanikgil, Y. Effects of preservatives in noseformulations on the mucosal integrity: an electron microscopic study. Pharmacology, 2004, 72(2), 113-120. [150] Marple, B.; Roland, P.; Benninger, M. Safety review of benzalkonium chloride used as a preservative in intranosesolutions: an overview of conflicting data and opinions. Otolaryngol. Head Neck Surg., 2004, 130(1), 131-141. [151] Szabadka, H. Clinical observations with Paxirasol nosespray. Ther. Hung., 1992, 40(1), 31-36. [152] Abe, K.; Irie, T.; Uekama, K. Enhanced nosedelivery of luteinizing hormone releasing hormone agonist buserelin by oleic acid solubilized and stabilized in hydroxypropyl-????cyclodextrin. Chem. Pharm. Bull. (Tokyo), 1995, 43(12), 2232-2237. [153] Park, J.S.; Oh, Y.K. ; Yoon, H.; Kim, J. M.; Kim, C.K. In situ gelling and mucoadhesive polymer vehicles for controlled intranosedelivery of plasmid DNA. J. Biomed. Mater. Res., 2002, 59(1), 144-151. [154] Mustafa, F.; Yang, T.; Khan, M.A.; Ahsan, F. Chain lengthdependent effects of alkylmaltosides on noseabsorption of enoxaparin. J. Pharm. Sci., 2004, 93(3), 675-683. [155] Z. Shao, A. K. Mitra, Bile salt-fatty acid mixed micelles as noseabsorption promoters. III. Effects on nosetransport and enzymatic degradation of acyclovir prodrugs. Pharm . Res ., 1994, 11(2), 243- 250. [156] Tengamnuay, P.; Mitra, A. K. Bile salt-fatty acid mixed micelles as noseabsorption promoters of peptides. II. In vivo noseabsorption of insulin in rats and effects of mixed micelles on the morphological integrity of the nosemucosa. Pharm Res., 1990, 7(4), 370-375. [157] Gonda, I.; Gipps, E. Model of disposition of drugs administered into the human nosecavity. Pharm. Res., 1990, 7(1), 69 75. [158] Li, L.; Nandi, I.; Kim, K.H.b Development of an ethyl laurate-based microemulsion for rapid-onset intranosedelivery of diazepam. Int. J. Pharm., 2002, 237(1-2), 77-85. [159] Zhang, Q.; Jiang, X.; Jiang, W.; Lu, W.; Su, L.; Shi, Z. Preparation of nimodipineloaded microemulsion for intranosedelivery and evaluation on the targeting efficiency to the brain. Int. J. Pharm., 2004, 275(1-2), 85-96. [160] Jogani, V.V.; Shah, P.J.; Mishra, P.; Mishra, A.K.; Misra, A.R. IntranoseMucoadhesive Microemulsion of Tacrine to Improve Brain Targeting. Alzheimer Dis. Assoc. Disord., 2008, 22(2), 116- 124. [161] Vyas, T.K.; Babbar, A.K.; Sharma, R.K.; Shashi, S.; Misra, A. Intranosemucoadhesive microemulsions of clonazepam: preliminary studies on brain targeting, J. Parm. Sci., 2006, 95(3), 570-80. [162] Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-tobrain delivery of drugs. Int. J. Pharm. 2009, 379(1), 146-57. [163] Pisal, S.S.; Paradkar, A.R.; Mahadik, K.R.; Kadam, S.S. Pluronic gels for nosedelivery of Vitamin B12. Part I: preformulation study. Int. J. Pharm., 2004, 270(1-2), 37-45. [164] Ugwoke, M.I.; Kaufmann, G.; Verbeke, N.; Kinget, R. Intranosebioavailability of apomorphine from carboxymethylcellulose-based drug delivery systems. Int. J. Pharm., 2000, 202(1-2), 125-131. [165] Vyas, T.K.; Babbar, A.K.; Sharma, R.K.; Shashi, S.; Misra, A. Preliminary braintargeting Studies on IntranoseMucoadhesive Microemulsions of Sumatriptan. AAPS PharmSciTech. 200, 7(1), E49-E57, Article 8, DOI: 10.1208/PT070108 (Accessed on 8th March 2010). [166] Majithiya, R.J.; Ghosh, P.K.; Umrethia, M.L.; Murthy , R.S.R. Thermoreversiblemucoadhesive Gel for NoseDelivery of Sumatriptan. AAPS PharmSciTech. 2006, 7(3), Article 67, DOI: 10.1208/pt070367 (Accessed on 8th March 2010). [167] Chand, R.; Naik, A.A.; Nair, H.A. Thermoreversible biogels for intranosedelivery of rizatriptan benzoate. Indian J. Pharm. Sci., 2009, 71(6), 723-725. [168] Aurora J. Development of NoseDelivery Systems: A Review. Drug Deliv. Technol., 2002, 2(7), 1-8. [169] Illum, L.; Farraj, N.F.; Davis, S.S. Chitosan as a novel nose delivery system for peptide drugs. Pharm. Res., 1994, 11(8), 1186-9. [170] Illum, L. Nosedrug delivery: new developments and strategies. Drug Discov. Today, 2002, 7(23), 1184-1189. [171] Edman, P.; Bjork, E.; Ryden, L.; Microspheres as a nosedelivery system for peptide drugs. J. Control. Release, 1992, 21(1-3), 165-172. [172] Illum, L.; Fisher, A.N.; Jabbal-Gill, I.; Davis, S.S. Bioadhesive starch microspheres and absorption enhancing agents act synergistically to enhance the noseabsorption of polypeptides. Int. J. Pharm., 2001, 222(1), 109-119. [173] Illum, L.; Farraj, N.F.; Fisher, A.N.; Gill, I.; Miglietta, M.; Benedett, L.M. Hyaluronic acid ester microspheres as a nosedelivery system for insulin. J. Control. Release, 1994, 29(12), 133- 141. [174] Illum, L.; Farraj, N.; Critchley, H.; Davis, S.S. Noseadministration of gentamicin using a novel microsphere delivery system. Int. J. Pharm., 1988, 46(3), 261-265. [175] Ryden, L.; Edman, P. Effect of polymers and microspheres on the noseabsorption of insulin in rats. Int. J. Pharm., 1982, 83(1-3), 1- 10. [176] Illum, L.; Jorgensen, H.; Bisgaard, H.; Krogsgaard, O.; Rossing, N. Bioadhesive microspheres as a potential nosedrug delivery system. Int. J. Pharm., 1987, 39(3), 189-199. [177] Shaji, J.; Poddar, A.; Iyer, S. Brain-targeted noseclonazepam microspheres. Indian J. Pharm. Sci., 2009, 71(6), 715-8. [178] Rassu, G.; Gavini, E.; Jonassen, H.; Zambito, Y.; Fogli, S.; Breschi, M.C.; Giunchedi, P. New chitosan derivatives for the preparation of rokitamycin loaded microspheres designed for ocular or noseadministration. J. Pharm. Sci., 2009, 98(12), 4852-4865. [179] Lim, S.T.; Forbes, B.; Martin, G.P.; Brown, M.B. In Vivo and In Vitro Characterization of Novel Microparticulates Based on Hyaluronan and Chitosan Hydroglutamate. AAPS PharmSciTech, 2001, 2(4), 20. [180] Krauland, A.H.; Guggi, D.; Bernkop-Schnurch, A. Thiolated chitosan microparticles: a vehicle for nosepeptide drug delivery. Int. J. Pharm., 2006, 307(2), 270-277. [181] Bhanushali, R.S.; Gatne, M.M.; Gaikwad, R.V.; Bajaj, A.N.; Morde, M.A. Nanoemulsion based intranosedelivery of antimigraine drugs for nose to brain targeting. Indian J. Pharm. Sci., 2009, 71(6), 707-709. [182] Wattanathorn, J.; Phachonpai, W.; Priprem, A.; Suthiparinyanont, S. Intranoseadministration of quercetin liposome decreases anxiety- like behavior and increases spatial memory. Am. J. Agri. & Biol., 2007, 2(1), 31-35. [183] Frey, W.H. Method for administering fibroblast growth factor to the brain. US Patent 6342478, http://www.wikipatents.com/USPatent- 6342478/method-for-administeringfibroblast-growthfactor- to-the-brain (Accessed on 8th March 2010). [184] Vyas, S.P.; Goswami, S.K.; Singh, R. Liposomes based nosedelivery system of nifedipine: Development and characterization. Int. J. Pharm., 1995, 118(1), 23-30. [185] Ahn, B.N.; Kim, S.K.; Shim, C.K. Preparation and evaluation of proliposomes containing propranolol hydrochloride. J Microencapsul., 1995, 12(4), 363-75. [186] Wang, X. ; Chi, N.; Tang, X. Preparation of estradiol chitosan nanoparticles for improving noseabsorption and brain targeting. Eur. J. Pharm. Biopharm., 2008, 70(3), 735740. [187] Bhavna; Sharma, V.; Ali, M.; Baboota, S.; Ali, J. Preparation and characterization of chitosan nanoparticles for nose to brain delivery of a cholinesterase inhibitor. Indian J. Pharm. Sci., 2007, 69(5), 712-713. [188] Vila, A.; Sanchez, A.; Janes, K.; Behrens, I.; Kissel, T.; Vila Jato J.L.; Alonso, M.J. Low molecular weight chitosan nanoparticles as new carriers for nosevaccine delivery in mice. Eur. J. Pharm. Biopharm., 2004, 57(1), 123-131. [189] Khatri, K.; Goyal, A.K.; Gupta, P.N.; Mishra, N.; Vyas, S.P. Plasmid DNA loaded chitosan nanoparticles for nosemucosal immunization against hepatitis B. Int. J. Pharm., 2008, 354(1-2), 235-241. [190] Vila, A.; Sanchez, A.; Evora, C.; Soriano, I.; Vila Jato, J.L.; Alonso, M.J. PEG-PLA nanoparticles as carriers for nosevaccine delivery. J. Aerosol Med., 2004, 17(2), 174-85. [191] Amidi, M.; Romeijn, S.G.; Verhoef, J.C.; Junginger, H.E.; Bungener, L.; Huckriede, A,; Crommelin, D.J.; Jiskoot, W. N-trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranosevaccination: biological properties and immunogenicity in a mouse model. Vaccine, 2007, 25(1), 144-153. [192] Gao, X.; Tao, W.; Lu, W.; Zhang, Q.; Zhang, Y.; Jiang, X.; Fu, S. Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranoseadministration. Biomaterials, 2006, 27(18), 3482-3490. [193] Hu, K.L.; Ni, Me.; Liang, F.; Jiang, X.G. Hydrophilic NoseGel of Lidocaine Hydrochloride: 2nd Communication: Improved bioavailability and brain delivery in rats with low ciliotoaricity. Arzneimittel- Forschung, 2009, 59(12), 635-640. [194] Mehta, M.R.; Surve, S.A.; Menon, M.D. Novel noseIn situ gelling system for treatment of sinusitis. Indian J. Pharm. Sci., 2009, 71(6), 721-722. [195] Torchilin, V.P. Structure and design of polymeric surfactant- based drug delivery systems. J Control. Release, 2001, 73(2-3), 137-72. [196] Tengamnuay, P.; Mitra, A.K. Bile salt-fatty acid mixed micelles as noseabsorption promoters of peptides. I. Effects of ionic strength, adjuvant composition, and lipid structure on the noseabsorption of [D-Arg2]kyotorphin. Pharm. Res., 1990, 7(2), 127-33. [197] Tengamnuay, P.; Mitra, A.K. Bile salt-fatty acid mixed micelles as noseabsorption promoters of peptides. II. In vivo noseabsorption of insulin in rats and effects of mixed micelles on the morphological integrity of the nosemucosa. Pharm. Res., 1990, 7(4), 370375. [198] Jain, R.; Nabar, S.; Dandekar, P.; Patravale, V. Micellar Nanocarriers: Potential Noseto-Brain Delivery of Zolmitriptan as Novel Migraine Therapy. Pharm. Res., 2010, 27(4), 655-664. [199] Bertram, U.; Bodmeier, R. In situ gelling, bioadhesive noseinserts for extended drug delivery: In vitro characterization of a new nosedosage form. Eur.J. Pharm. Sci., 2006, 27(1), 62-71. [200] Bertram, U.; Bernard, M.C.; Haensler, J.; Maincent, P.; Bodmeier, R. In situ gelling noseinserts for influenza vaccine delivery. Drug Dev. Ind. Pharm., 2010, 36(5), 581-93. [201] Hardy, J.G.; Lee, S.W.; Wilson, C.G. Intranose drug delivery by spray and drops. J .Pharm. Pharmacol., 1985, 37(5), 294-297. [202] Aoki, F.Y.; Crowley, J.C. Distribution and removal of human serum albumintechnetium 99m instilled intranasally. Br. J. Clin. Pharmacol., 1976, 3(5), 869-878. [203] Tsikoudas, A.; Homer, J.J. The delivery of topical nosesprays and drops to the middle meatus: a semiquantitative analysis. Clin. Otolaryngol., 2001, 26(4), 294-297. [204] Bryant, M.L., Brown, P. ; Gurevich, N.; McDougall, I.R. Comparison of the clearance of radiolabelled nose drops and nosespray as mucosally delivered vaccine. Nucl. Med. Commun., 1999, 20(2), 171-174. [205] Chien, Y.W.; Su, K.S.E.; Chang, S.F. Physicochemical, biopharmaceutical, and toxicophysiological considerations. In: NoseSystemic Drug Delivery, 1989, Marcel Dekker: New York, pp. 39-90. [206] Mygind, N.; Vesterhauge , S. Aerosol distribution in the nose. Rhinology, 1978, 16(2), 79-88. [207] Daley-Yates, P.T.; Baker, R.C. Systemic bioavailability of fluticasone propionate administered as nosedrops and aqueous nosespray formulations. Br. J. Clin. Pharmacol., 2001, 51(1), 103-105. [208] Henry, R.J. ; Ruano, N.; Casto, D.; Wolf, R.H. A pharmacokinetic study of midazolam in dogs: nosedrop vs. atomizer administration. Pediatr. Dent., 1998, 20(5), 321-326. [209] BD Accuspray™, Nosespray system, http://www.bd.com/pharmaceuticals/products/nasal-spray.asp (Accessed on 8th March 2010). [210] Intelligent nosedrug delivery with ViaNase ID, http://www.kurvetech.com/devices_vianaseid.asp (Accessed on 8th March 2010). [211] Novel nosedrug delivery device, http://www.optinose.com (Accessed on 8th March 2010). [212] DirectHaler Nasal, http://www.directhaler.com/frame.cfm/cms/ sprog=1/grp=6/menu=1/ (Accessed on 8th March 2010). [213] Needle-free intranosedrug delivery with Mad Nasal, http://www.wolfetory.com/nasal.php (Accessed on 8th March 2010). [214] Gomedical nose spray, http://www.gomedical.com.au/products/nasalspray.php Gomedical. com.au (Accessed on 8th March 2010). [215] T. Wolfe. IntranoseFentanyl for Acute Pain: Techniques to Enhance Efficacy. Ann. Emerg. Med., 2007, 49(5), 721-722. [216] Huge, V.; Lauchart, M.; Magerl , W.; Schelling, G.; Beyer, A.; Thieme, D.; Shahnaz, C. Azad. Effects of low-dose intranose(S)- ketamine in patients with neuropathic pain. Eur. J. Pain, 2010, 14(4), 387-394. [217] Frey, W.H. Therapy procedure for drug delivery for trigeminal pain. WO/2007/025286, http://www.wipo.int/pctdb/en/wo.jsp?wo=2007025286 (Accessed on 8th March 2010). [218] Rapoport, A.M.; Bigal, M.E.; Tepper, S.J.; Sheftell, F.D. Intranosemedications for the treatment of migraine and cluster headache. CNS Drugs, 2004, 18(10), 671-85. [219] Wang, S.J.; Fuh, J.L.; Wu, Z.A. Intranosesumatriptan study with high placebo response in Taiwanese patients with migraine. J. Chin. Med. Assoc., 2007, 70(2), 39-46. [220] Tiwari, N.G.; Bajaj, A.N. Formulation development of eucalyptus oil microemulsion for intranosedelivery. Indian J. Pharm. Sci., 2007, 69(5), 731-733. [221] Hanson , L.R.; & Frey II, W.H. Strategies for intranosedelivery of therapeutics for the prevention and treatment of neuroAIDS. J. Neuroimmune Pharm., 2007, 2(1), 81-86. [222] Ruff, M.R.; Polianova, M.; Yang, Q.; Leoung, G.S.; Ruscetti, W.F.; Pert, C.B. Update on D-Ala-Peptide T-Amide (DAPTA): A viral entry inhibitor that blocks CCR5 Chemokine Receptors. Curr.HIV Res., 2003, 1(1), 51-67. [223] Tomotaka, S.; Toshiyasu, S.; Shinji, Y.; Hitoshi, S.; Yoshiharu, T.; Shobu, S. Transnosedelivery of anticancer drugs to the brain tumor. A new strategy for brain tumor chemotherapy. Drug Deliv. Syst., 1999, 14(5), 365-371. [224] Tomotaka S.; Hidalgo, I.J.; Furubayashi, T.; Katsumi, H.; Sakane, T.; Yamamoto, A. ; Yamashita, S. The transnosedelivery of 5- fluorouracil to the rat brain is enhanced by acetazolamide (the inhibitor of the secretion of cerebrospinal fluid. Int. J.Pharm., 2009, 377(1-2), 85-91. [225] Dennis, F.; Frey II, W.H.; Gryaznov, S. CNS-tumor treatment method and composition. WO 2007127163, http://www.wipo.int/pctdb/en/wo.jsp?KEY=07%2F127163&IA=US2007009839&DISPLAY=CLAIMS (Accessed on 8th March2010). [226] Wang, D.; Gao, Y.; Yun, L. Study on brain targeting of raltitrexed following intranoseadministration in rats. Cancer Chemother. Pharmacol., 2006, 57(1), 97-104. [227] Hanson, L.R.; Frey II, W.H. Intranosedelivery bypasses the bloodbrain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci., 2008, 9(Suppl 3), S5. [228] Kumar, M.; Misra, A.; Babbar, A.K.; Mishra, A.K.; Mishrahttp://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T7W-4S50K5P- 1&_user=10&_coverDate=06%2F24%2F2008&_rdoc=1&_fmt=high&_orig=search&_sort=d&_docanchor=&view=c&_searchStrId1275489134&_rerunOrigin=google&_acct=C000050221&_version =1&_urlVersion=0&_userid=10&md5=3b619fdacf7658397f9adaa ef4d752b9 - aff3, P.; Pathak, K. Intranosenanoemulsion based brain targeting drug delivery system of risperidone. Int. J.Pharm., 2008, 358(1-2), 285-291. [229] Benedict, C.; Scheller, J.; Rose-John, S.; Born, J.; Marshall, L. Enhancing influence of intranoseinterleukin-6 on slow-wave activity and memory consolidation during sleep. FASEB J., 2009, 23, 3629-3636. [230] Yu, Y.P.; Xu, Q.Q.; Zhang, Q.; Zhang, W.P.; Zhang, L.H.; Wei, E.Q. Intranoserecombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci. Lett., 2005, 387(1), 5-10. [231] Wang, Z.L.; Cheng, S.M.; Ma, M.M.; Ma, Y.P.; Yang, J.P.; Xu, G.L.; Liu, X.F. Intranasally delivered bFGF enhances neurogenesis in adult rats following cerebral ischemia. Neurosci. Lett., 2008, 46(1), 30-35. [232] Wermeling, D.P. Intranose Benzodiazepine compositions. WO/2005/089768, http://www.wipo.int/pctdb/en/wo.jsp?wo=2005089768 (Accessed on 8th March 2010). [233] Jeannet, P.Y.; Roulet, E.; Maeder-Ingvar, M.; Gehri, M.; Jutzi, A.; Deonna, T. Home and hospital treatment of acute seizures in children with nosemidazolam. Eur J. Paediatr. Neurol., 1999, 3(2), 73-77. [234] de Haan, G.J.; van der Geest, P.; Doelman, G.; Bertram. E.; Edelbroek, P. A comparison of midazolam nosespray and diazepam rectal solution for the residential treatment of seizure exacerbations. Epilepsia, 2010, 51(3), 478-82. [235] Porecha, S.; Shah, T.; Jogani, V.; Naik, S.; Misra, A. Microemulsion based intranosedelivery system for treatment of insomnia. Drug Deliv., 2009, 16(3), 128-134. [236] Gregory, C.W.; Smith, P.S. Leuprolide acetate and acetylcholinesterase inhibitors or nmda receptor antagonists for the treatment of alzheimer’s disease. WO/2006/071274, http://www.wipo.int/pctdb/en/wo.jsp?wo=2006071274 (Accessed on 8th March 2010). [237] Wearly, L.L. Recent progress in protein and peptide delivery by noninvasive routes. Crit. Rev. Ther. Drug Carrier Syst., 1991, 8(4), 331-94. [238] Misra, A.; Jogani, V.; Jinturkar, K.; Vyas, T. Recent Patents Review on IntranoseAdministration for CNS Drug Delivery. Recent Patents on Drug Delivery & Formulation, 2008, 2 (1), 25-40. [239] Frey, W.H.; Liu, J.; Chen, X.; Thorne, R.G.; Fawcett, J.R.; Ala, T.A. Delivery of 125INGF to the brain via the olfactory route. Drug Deliv., 1997, 4, 87-92. [240] Chen, X.Q.; Fawcett, J.R.; Rahman, Y.E.; Ala, T.A.; Frey, W.H. Delivery of nerve growth factor to the brain via the olfactory pathway. J. Alzheimers Dis., 1998, 1(1), 35-44. [241] Frey, W.H.; Thorne, R.G.; Pronk, G. Delivery of Insulin like growth factor-1 to the brain and spinal cord along olfactory and trigeminal pathways following intranoseadministration: a noninvasive method for bypassing the blood brain barrier. Soc Neurosci. (Abstract), 2000, 26, 1365-1370. [242] Kucheryanu, V.G.; Kryzhanovsky, G.N. ; Kudrin, V.S.; Yurasov, V.V.; Zhigaltsev, I.V. In: Intranosefibroblast growth factors: delivery into the brain exerts antiparkinsonian effect in mice. Proceedings of the 26th Internl. Symposium on controlled release of bioactive materials, Torchilin V, Veronese FM, Eds. Boston MA, Controlled release society Inc, 1999, pp 643. [243] Gozes, I.; Bardea, A.; Reshef, A.; Zamoostiano, R.; Zhukovsky, S.; Rubinraut, S.; Fridkin, M.; Brenneman, D.E. Neuroprotective strategy for Alzheimer disease: intranoseadministration of a fatty neuropeptide. Proc. Natl. Acad. Sci., 1996, 93(1), 427-432. [244] Pietrowsky, R.; Struben, C.; Molle, M.; Fehm, H.L.; Born, J. Brain potential changes after intranoseadministration vs. Intravenous administration of vasopressin: evidence for a direct nose-brain pathway for peptide effects in humans. Biol. Psychiatry, 1996, 39(5), 332340. [245] Pietrowsky, R.; Thieman, A.; Kern, W.; Fehm, H.L.; Born, J.A. A nose-brain pathway for psychotropic peptides: evidence from a brain evoked potential study with cholecystokinin. Psychoneuroendocrinology, 1996, 2(6), 559-572. [246] Smolnik, R.; Molle, M.; Fehm, H.L.; Born, J . Brain potentials and attention after acute and subchronic intranoseadministration of ACTH4-10 desacetyl-a-MSH in humans. Neuroendocrinol, 1999, 70(1), 63-72. [247] Fehm, H.L.; Smolnik, R.; Kern, W.; McGregor, G.P.; Bickel, U.; Born, J. The melanocortin melanocyte-stimulating hormone/ adrenocotropin (4-10) decreases body fat in humans. J Clin Endocrinol Metab, 2001, 86, 1144-1148. [248] Kern, W.; Born, J.; Schreiber, H.; Fehm, H.L. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes, 1999, 48(3), 557-563. [249] Hinchcliffe, M.; Illum, L. Intranose insulin delivery and therapy. Adv. Drug Deliv. Rev., 1999, 35(2-3), 199-234. [250] Hussain, A.A. Intranosedrug delivery. Adv. Drug Deliv. Rev.,1998, 29(1-2), 39-49. [251] Ross, T.M.; Zuckermann,R.N.; Reinhard, C.; Frey II, W.H. Intranoseadministration delivers peptoids to the rat central nervous system. Neurosci. Lett., 2008, 439(1), 30-33. [252] Danielyan, L.; Schafer, R.; von Ameln-Mayerhofer, A.; Buadze, M.; Geisler, J.; Klopfer, T.; Burkhardt, U.; Proksch, B.; Verleysdonk, S.; Ayturan, M.; Buniatian, G.H.; Gleiter, C.H.; Frey II, W.H. Intranosedelivery of cells to the brain. Eur. J. Cell Biol., 2009, 88(6), 315-324. [253] Jamie, T. IntranoseDelivery of Stem Cells Bypasses Blood-Brain Barrier. Neurology Today, 2009, 9(15), 11-13. [254] Vajdy, M.; O’Hagan, D.T. Microparticles for intranoseimmunization. Adv. Drug Deliv. Rev., 2001, 51(1-3), 127-141. [255] Oh, Y.K.; Kim, J.P.; Hwang, T.S.; Ko, J.J.; Kim, J.M.; Yang, J.S.; Kim, C.K. Noseabsorption and biodistribution of plasmid DNA: an alternative route of DNA vaccine delivery. Vaccine, 2001, 19(31), 4519-4525. [256] Midthun, K.; Steven, M. (2003-07-17). \"CBER Approval Letter, Influenza Virus Vaccine, Live, Intranose(FluMist)\". U.S. Food and Drug Administration. http://www.fda.gov/cber/approvltr/inflmed061703L.htm, Retrieved 2008-07-06 (Accessed on 8th March 2010). [257] Greenway, F.L.; Martin, C.K.; Gupta, A.K.; Cruickshank, S.; Whitehouse, J.; DeYoung, L.; Kamdar, K.; Caruso, M.K.; Roberts, A.T.; England, M.; Dumas, K.; Laidlaw, B.J.; Rogers, B.; Cowley, M.A. Using intranoselidocaine to reduce food intake. Int. J. Obes. (Lond), 2007, 31(5), 858-863. [258] Sharma, G.; Mishra, A.K.; Mishra, P.; Misra, A. Intranosecabergoline: pharmacokinetic and pharmacodynamic studies. AAPS PharmSciTech., 2009, 10(4), 1321-1330. [259] Hallschmid, M.; Benedict, C.; Schultes, B.; Fehm, H.L.; Born, J.; Kern, W. IntranoseInsulin Reduces Body Fat in Men but not in Women. Diabetes, 2004, 53(11), 30243029. [260] Kramer, R. Intranoseformulation of rotigotine. WO/2005/063236, http://www.wipo.int/pctdb/en/wo.jsp?WO=2005063236. (Accessed on 8th March 2010). [261] Ross, T.M.; Martinez, P.M.; Renner, J.C.; Thorne, R.G.; Hanson, L.R.; Frey II, W.H. Intranoseadministration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J. Neuroimmunol., 2004, 151(1-2), 66-77. [262] Billotte, A.; Dunn, P.J.; Henry, B.T.; Marshall, P.V.; Woods, J.J. Intranoseformulations for treating sexual disorders. EP0967214, http://www.freepatentsonline.com/EP0967214.html. (Accessed on 8th March 2010). [263] http://clinicaltrials.gov. (Accessed on 8th March 2010).
Copyright
Copyright © 2024 Rohan Pawar, Shubham Pawar . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET59316
Publish Date : 2024-03-22
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

