Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Short Review on Nutritional Management of Alzheimer Disease
Authors: Ms. Sonal R. Barde, Dr. Deepa S. Mandlik, Samina B. Sanadi, Ms. Pranali Bangaiya, Dr. Kanthale Sangameshwar
DOI Link: https://doi.org/10.22214/ijraset.2024.63797
Certificate: View Certificate
Abstract
Amyloid-(A) protein is densely deposited in the brain in Alzheimer\'s disease (AD), a neurodegenerative condition that also causes memory loss and dementia. There is neuronal destruction and deterioration of neural connections in the cerebral cortex region of the brain in Alzheimer\'s disease, as well as significant loss of brain mass. It is critical to review medication history, family history, and symptoms when diagnosing Alzheimer\'s dementia. Alzheimer\'s disease symptoms usually appear after the age of 65, but the types of Alzheimer disease that run-in families occur earlier. When Alzheimer\'s disease progresses to its later stages, a person\'s physical functioning is frequently impacted. This can manifest as problems with balance or weakness, as well as swallowing difficulties; all of these symptoms may have serious consequences. There is overwhelming evidence that suggests dietary factors may contribute to the onset of Alzheimer\'s disease and aging- related cognitive decline. Nutrition and diet are therefore crucial in reducing the risk of Alzheimer\'s disease. The brain may be protected from oxidative and inflammatory damage by dietary supplements of antioxidants, polyunsaturated fatty acids, B complex vitamins, essential minerals, and polyphenols, according to epidemiological research. This review mainly focuses on nutritional management of Alzheimer disease.
Introduction
I. INTRODUCTION
Alzheimer's disease (AD) was first described in 1906 by Alois Alzheimer, a German neuropathologist. By the beginning of the twenty-first century, Alzheimer's disease (AD) was recognised as the most common form of dementia among geriatric people [1]. Alzheimer's disease (AD) is a progressive brain disease that causes 60-80% of dementia cases, according to the Alzheimer Association [2]. Often manifesting in mid- to late adulthood, AD is a neurodegenerative illness. There is neuronal destruction and deterioration of neural connections in the cerebral cortex region of the brain in Alzheimer's disease, as well as significant loss of brain mass. Within 5-10 years of onset, Alzheimer's disease is invariably progressive and fatal. There is neuronal destruction and deterioration of neural connections in the cerebral cortex region of the brain in Alzheimer's disease, as well as significant loss of brain mass [1]. AD is characterised by cognitive decline, affecting memory, thought, language, reasoning, learning, orientation, comprehension, judgement, behaviour, and daily activities and primarily causing dementia [3]. Alzheimer's disease (AD) is classified as a progressive neurodegenerative disease distinguished by diffuse cortical atrophy with three levels of severity: mild, moderate, and severe [4]. Alzheimer's disease must be distinguished from the following dementias: vascular dementia, dementia with Lewy bodies, Parkinson's disease with dementia, frontotemporal dementia, and reversible dementias [5].
It is critical to review medication history, family history, and symptoms when diagnosing Alzheimer's dementia. Some laboratory tests are also performed for the diagnosis of Alzheimer's dementia, such as brain imaging tests, which aid in distinguishing between different types of degenerative brain disease and ruling out other causes, such as haemorrhages, brain tumours, or strokes. Positron Emission Tomography (PET), Magnetic Resonance Imaging (MRI), and Computerized Tomography (CT) are the most commonly used brain-imaging technologies (CT) [6]. As a result, several non-pharmacological interventions are required to improve these patients' quality of life. Nutritionists, psychologists, physical therapists, speech therapists, and other professionals who can provide appropriate guidance for these patients' symptoms are therefore required. Weight loss and behavioural changes related to food are major scientific research topics for patients with Alzheimer's disease, as they cause a decline in the quality of life of both patients and carers. The purpose of this review is to demonstrate current nutritional treatment methods for Alzheimer's disease patients [4].
II. CAUSES AND RISK FACTORS
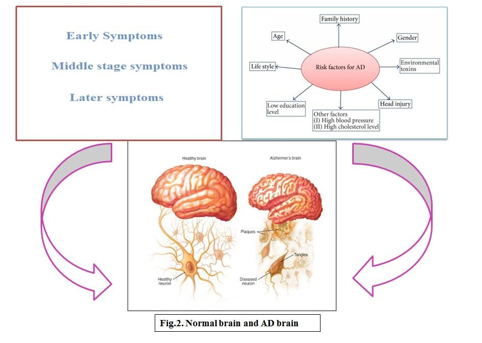
The aggregation of a normal protein, b-amyloid (Ab), within the neocortex is thought to be the cause of Alzheimer's disease (AD). Recent evidence suggests that abnormal interactions with neocortical metal ions, particularly Zn, Cu, and Fe, cause Ab precipitation and toxicity in AD [7]. The development of Alzheimer's disease is linked to two abnormal proteins in the brain known as amyloid and tau, both of which are toxic to nerve cells (neurons) in the brain. The accumulation of these proteins in neurons eventually leads to neuron death, impaired brain function, and dementia symptoms. Dementia is a term used to describe a decline in mental abilities such as memory, language, and logical reasoning. The precise cause of Alzheimer's disease is unknown. It usually runs in families, implying that there is a genetic component. More than 30 genes associated with Alzheimer's disease have been identified. Three genes have been discovered to be "dominantly" passed down from parent to child, with approximately half of the children of an affected parent eventually developing Alzheimer disease. Alzheimer's disease symptoms usually appear after the age of 65, but the types of Alzheimer disease that run-in families occur earlier [8].

- Hypotheses: There are numerous descriptive hypotheses regarding the causes of Alzheimer's disease, including the cholinergic hypothesis, amyloid hypothesis, tau propagation hypothesis, mitochondrial cascade hypothesis, calcium homeostasis hypothesis, neurovascular hypothesis, . However, the ultimate etiology of AD remains obscure [9, 10].
- Smoking: Smoking may be unrelated to the onset of Alzheimer's disease or may increase the risk of AD, they do not specifically address the potential long-term effects of nicotinic receptor modulation in AD (Newhouse et al 2000). Cigarette smoke contains numerous chemicals and substances, making it impossible to determine how nicotinic modulation alone might affect AD risk [11].
- Alcohol: Alcohol abuse is well known to cause alcohol dementia. Furthermore, middle-aged heavy drinkers, particularly apoE4 allele carriers, were found to have a more than threefold increased risk of dementia and Alzheimer's disease later in life [9]. Alcohol consumption causes morphological changes such as atrophy, with cognitive improvement after abstinence. Other research has linked morphological changes in the brain to the loss of a number of nerve cells in the white matter, which primarily consists of nerve fibres that connect neurons and/or cortical cholinergic neurons, which are known to be affected in Alzheimer's disease [12].
- Over weight and Obesity: Higher BMI in middle age is a risk factor for AD and other dementias [9]. Based on meta-analysis studies, patients with obesity or other metabolic diseases, have almost a two-fold greater risk of developing AD. Being overweight in midlife increases the risk of Alzheimer's disease, vascular dementia, or other forms of dementia by 35, 33, and 26%, respectively; obesity increases the risk even more [13].
- Physical Activity: Physical activity was inversely associated with risk of AD in most studies. Physical activity in the form of various leisure activities, rather than sports or specific physical exercise, was associated with a lower risk of dementia, Even walking may lower the risk of dementia and cognitive impairment [9]. Physical activity may also help to prevent Alzheimer's disease by increasing neurotrophic factors like BDNF (Brain Derived Neurotrophic Factor), IGF-1 (Insulin-Like Growth Factor), and VEGF (Vascular Endothelial Growth Factor), as well as by reducing free radicals in the hippocampus and increasing superoxide dismutase and eNOS (endothelial nitric oxide synthase)
- Mental Activity: Several large prospective studies conducted over the last decade have found that older people who engage in mentally stimulating activities on a regular basis are less likely to develop AD or experience cognitive decline [14]. Reading, social and cultural activities, knitting, gardening, dancing, tabletop games, playing musical instruments, and watching specific TV shows all showed a protective effect against dementia and Alzheimer's disease [9].
- Nutritional Factors: Nutritional factors play an important role in mental health. Eating balanced meals on a regular basis, as well as consuming nutrients for mental health such as omega-3 FAs, antioxidants, niacin, folate, vitamin B6, and vitamin B12 at recommended dietary intake levels, are especially recommended [15].
- Dibetes Mellitus: Epidemiological studies show a clear link between type 2 diabetes and an increased risk of developing Alzheimer's disease. Several mechanisms are proposed for this association, including insulin resistance and deficiency, impaired insulin receptor, hyperglycemia toxicity, adverse effects due to advanced glycation end products, cerebrovascular damage, vascular inflammation, and others [16]. Furthermore, borderline conditions, prediabetes, or impaired glucose tolerance are linked to an increased risk of Alzheimer's disease and other dementias in the elderly [9].
- Cardiovascular and Cerebrovascular Disease: Alzheimer's disease and cardiovascular disease (CVD) share significant cardiometabolic and lifestyle risk factors that occur in middle-aged to elderly populations. Both Alzheimer's disease and cardiovascular disease are associated with advancing age, and both are among the leading causes of death. Coronary heart disease (CHD), hypertension, stroke, and heart failure are the most common causes of CVD. These diseases are frequently linked and share an atherosclerotic pathology. Studies to identify modifiable risk factors for Alzheimer's disease have focused on all known risk factors for atherosclerosis [9, 17]. Cerebrovascular changes such as hemorrhagic infarctions, small and large ischemic cortical infarctions, vasculopathies, and changes in cerebral white matter have all been linked to an increased risk of dementia [16].
- Hypercholesterolemia and Statin Therapy: Hypercholesterolemia raises the risk of Alzheimer's disease primarily through its effects on the blood-brain barrier. Elevated circulating cholesterol levels have been shown in studies to compromise the integrity of the blood-brain barrier. A number of studies have been conducted to test the hypothesis that statins, which play a role in cholesterol reduction, may prevent the onset or progression of AD. Early epidemiological studies in this area predicted that statins could reduce the incidence of Alzheimer's disease by up to 70% [16, 17].
- Blood pressure and Mangement of Hypertension: Blood pressure (BP) level is a commonly investigated vascular risk factor. Longitudinal studies of the relationship between midlife blood pressure and later Alzheimer's disease have discovered that high blood pressure predicts the development of Alzheimer's disease (AD) [18]. Variability in systolic blood pressure (SBP) has been linked to dementia, and greater variability was found to be a predictor of faster disease progression in Alzheimer's disease (AD) [19]. Hypertension can cause changes in the vascular walls, which can lead to hypoperfusion, ischemia, and cerebral hypoxia, all of which can contribute to the development of Alzheimer's disease [16].
- Social Network and social Engagement: Low social engagement in older adults who are cognitively normal but have evidence of AD pathophysiologic change may be a marker of neurocognitive vulnerability. Understanding changes in social engagement in older adults may lead to earlier Alzheimer's disease diagnosis and advancements in evidence-based prevention and treatment [20].
- Education and Socioeconomic Status: Lower education is associated with an increased risk of dementia and Alzheimer's disease [9]. Other than educational quality, social and contextual factors, as well as racial residential segregation, have been linked to an increased risk of developing Alzheimer's disease [21].
III. COMPLICATION
When Alzheimer's disease progresses to its later stages, a person's physical functioning is frequently impacted. This can manifest as problems with balance or weakness, as well as swallowing difficulties; all of these symptoms may have serious consequences. As a result, in order to maintain the patient's well-being and keep them safe from harm, it is critical to be aware of the various complications that may result from Alzheimer's disease's consequences.
- Depression: Depression is one of the most common psychiatric complications of Alzheimer's disease, affecting up to 50% of patients. According to epidemiologic data, mood symptoms are more common in mild to moderate dementia and less common in severe dementia. The decline in advanced dementia may be related to the difficulty in assessing depression in severe Alzheimer disease. Although depressive symptoms fluctuate over time, at least one long-term follow-up study found that depression persists, with a 30% to 40% chance of persistence every 6 months. The consequences of depression in Alzheimer's disease highlight its significance. Because depression in Alzheimer's disease is a potentially treatable condition, treatment may be associated with reversal of its consequences [22].
- Agitation: Agitation is observed in 20% to 50% of people with moderate-to-severe Alzheimer's disease (AD). Agitation is linked to lower quality of life, increased carer burden, and higher rates of institutionalisation and mortality. Non-pharmacologic interventions are considered first-line therapy for the treatment of agitation in Alzheimer's disease. However, when non - pharmacologic interventions fail to relieve severe agitation, judicious use of pharmacologic interventions is advised [23].
- Psychotic Feature: The concept of Alzheimer's disease and dementia psychosis is developed in terms of prevalence, incidence, clinical characteristics, clinical course, and potential response to treatment. Psychosis is a common complication of dementia. During the course of their illness, up to 90% of dementia patients develop significant behavioural problems. Substantial proportion of patients with AD develop delusions or hallucinations sometime over the course of their illness [24].
- Dehydration and Malnutrition: Dysphagia, or swallowing impairment, is a growing concern in Alzheimer's disease (AD) patients, leading to malnutrition, dehydration, weight loss, functional decline, fear of eating and drinking, and a decrease in quality of life. Thus, diagnosing dysphagia in AD patients is critical to ensuring effective management, avoiding complications, and lowering comorbidity and mortality in such a growing population. Dysphagia is a common issue in the elderly population. So apart from old age, a variety of other health conditions, such as neurological diseases, stroke, and dementia, can contribute to the development of dysphagia in this population. As a result, advanced age combined with cognitive disorders predisposes to dysphagia susceptibility [25].
- Pneumonia: Pnemonia is a major, complicated disease in patients with AD. A recent meta-analysis found that people with dementia had an increased risk of pneumonia-related death. Using neuropathological dementia diagnoses, the study discovered that patients with all three major subtypes of dementia had a high incidence of pneumonia complications. Alzheimer's disease (AD), dementia with Lewy bodies (DLB), and vascular dementia are the three major types of dementia (VaD). For AD and DLB, the median total survival time from dementia onset was 8 years, and for VaD, it was 5 years. Patients with VaD had a shorter life expectancy than those with AD or DLB. Male gender, pneumonia complications, diabetes mellitus, being 75 years old at the time of onset, and VaD were all associated with a shorter survival time among dementia patients [26].
IV. PATHOPHYSIOLOGY
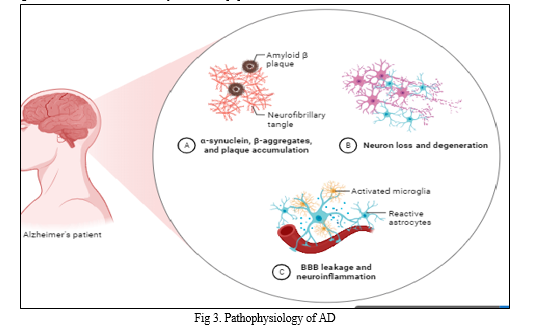
Alzheimer's disease (AD) is characterised by the development of extracellular plaques of the insoluble β-amyloid peptide (Aβ) and neurofibrillary tangles (NFT) of tau in the cytoplasm of neurons [16]. The pathology of Alzheimer's disease can be broken down into three major chapters: lesions related to accumulation (also known as "positive lesions"), lesions related to losses (also known as "negative lesions"), and finally lesions related to reactive processes (inflammation and plasticity) [27]. An imbalance between the production and clearance of Aβ peptide is caused by β and γ-secretases. As a result, Aβ peptides spontaneously coalesce into fibrils with an insoluble beta- sheet conformation, aggregate into soluble oligomers, and eventually deposit in diffuse senile plaques [28]. The production and release of proinflammatory cytokines like IL-1B, TNF-a, and IFN-y occur as a result of microglial activation. These cytokines then cause the astrocyte- neuron in the area to produce more AB42 oligomers, causing an increase in AB42 synthesis and distribution [29]. A42 oligomers are created by the cooperative actions of the associated astrocyte and neurons. A42oligomers have been found to cause oxidation damage, promote tau hyperphosphorylation, and have a toxic effect on synapses and mitochondria.[28]. The enzymatic proteolysis of amyloid precursor protein (APP), a naturally occurring protein that is crucial for maintaining the homeostasis of the brain, yields the A peptide, which has 36 to 43 amino acids [16].
V. ALLOPATHIC REMEDIES
A. Donepezil, Galantamine, and Rivastigmine
Acetylcholinesterase inhibitors (AChEIs) such as donepezil, galantamine, and rivastigmine, prevent the breakdown of acetylcholine (ACh) in the synapses, enhancing cholinergic neurotransmission and reducing cognitive decline in patients with mild to moderate AD over the short and long terms. However, the adverse effects of acetylcholinesterase inhibitors (ACEIs), which include nausea, diarrhoea, cramping, and reduced heart rate, are dose-limiting [30].
B. Memantine
In patients with moderate to severe AD, NMDA receptor antagonists, such as c, reduce the decline in cognitive function. Other studies have demonstrated improved cognitive benefits and a decreased rate of clinical deterioration in AD patients receiving donepezil and memantine combined therapy. Evidence exists for the harmful effects of memantine, including somnolence [30]. Memantine therapy decreased irritability, hostility, and problems with hunger and eating. Memantine lessened patients' anxiety and hostility [31]
C. Pioglitazone
Whilst also lowering peripheral insulin and improving insulin sensitivity, pharmacologic treatment with thiazolidinedions, such as rosiglitazone and pioglitazone, agonists of the nuclear receptor peroxisome proliferator-activated receptor (PPAR-), may provide some therapeutic relief for AD. Inhibiting the expression of inflammatory genes, changing A homeostasis, and having neuroprotective effects have also been demonstrated for PPAR-agonists.[32]
D. Dopamine, Serotonin, and Epinephrine
Monoamine neurotransmitters that are broken down in the central nervous system (CNS) by monoamine oxidases (MAOs) (Cai, 2014). Rasagiline is a medication that has been clinically approved to treat the symptoms of Parkinson's disease. It works by inhibiting MAOB, the predominant isoform in the human brain. It was studied clinically as an adjunctive treatment for AD alongside donepezil.[33]
E. Semagacestat
The enzymatic intramembrane cleavage of APP by the high-molecular weight complex known as -secretase results in the generation of A. Presenilin, Nicastrin, the Anterior Pharynx, and Presenilin Enhancer-2 are at least four of the subunit proteins that make up -secretase. Presenilins are of exceptional pathophysiological significance because they are known to contain over 150 autosomal dominant point mutations that all result in aggressive early-onset AD. An increased A42/A40 is the result of these mutations [1014693]. As a result, it seems logical to prevent A from building up in the brains of AD patients by inhibiting the catalytic unit (presenilin) of the -secretase enzymatic complex. Three of these chemicals have an impact on CSF A levels, a possible disease biomarker. These substances are semagacestat [1010969] by Eli Lilly & Co., BMS-708163 by Bristol-Myers Squibb Co., and MK-0752 by Merck & Co Inc. Semagacestat is the most well- researched and sophisticated of these substances [34].
VI. NUTRITIONAL MANAGEMENT
|
Sr.no |
Nutrition |
Activities |
Ref. |
|
1. |
Antioxidant |
There are oxidative and inflammatory processes involved in Alzheimer's disease. When the intracellular capacity to eliminate free radicals is exceeded, oxidative stress results, altering DNA, lipids, polysaccharides, and proteins as well as the homeostatic redox balance. The majority of cases of AD are late-onset sporadic forms, and there is mounting evidence that oxidative stress plays a significant role in these cases. Alzheimer's disease has been linked to abnormally high levels of oxidative stress in both the blood and brain. Reduced antioxidant defences, amyloid-toxicity-related toxicity, and/or altered metal metabolism in the brain and peripheral tissues have all been linked to changes in Alzheimer's disease that result in a prooxidative imbalance. |
[35 ,36] |
|
2. |
Dietary Fat |
The development of Alzheimer's disease and changes in neuronal integrity during ageing are significantly impacted by changes in fatty acid composition and levels. |
[35] |
|
1.Saturated Fat and Trans Fat |
Saturated and transunsaturated fat intake increased the risk of AD. AD and dietary cholesterol, total fat, or animal fat. 52 Higher intakes of total fat, saturated fat, and cholesterol were linked to an increased risk of elevated chance of dementia, |
[42] |
|
|
2.Monounsaturated fatty acid (MUFA) |
Higher consumption of monounsaturated fatty acids was linked to improved cognitive performance, while higher consumption of saturated fatty acids was associated with worse cognitive performance. |
[35] |
|
|
3.Polyunsaturated Fatty Acid (PUFA |
Long-term -3 supplementation to animal models of Alzheimer's disease revealed that a decreased -6/-3 ratio decreased A, prevented neuronal failure, and improved cognitive function. The main omega-3 long-chain PUFAs (omega-3 LC-PUFAs) are docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which regulate the excitability of the neuronal membrane and boost the capacity for neuronal transmission, enhancing memory and learning. |
[35] |
|
|
3. |
Carbohydrate |
Possession of one or more alleles of the epsilon-4 variant (E4) of the apolipoprotein E gene is a risk factor for late-onset AD. In populations that have long histories of exposure to agriculture, E4 is rare, according to a meta-analysis of allele frequencies, which suggests that a high-carbohydrate (HC) diet may have selected for it. opposed to E4 carriers. A common mechanism for the aetiology of AD is suggested by the fact that the apoE4 protein alters lipid metabolism in a manner similar to that of an HC diet. Two general mechanisms are proposed to explain why HC diets that are out of step with evolution are the main cause of AD. Imbalances in the central nervous system's lipid metabolism prevent the function of membrane proteins like the amyloid precursor protein and glucose transporters. In cerebral neurons, prolonged, excessive insulin/IGF signalling speeds up cellular damage. These two elements ultimately cause AD to develop clinically and pathologically. |
[41] |
|
4. |
Vitamins |
Vitamins are strong antioxidants. The environment in the brain becomes more and more contaminated with free radicals, which causes a steady decline in cognitive function that worsens dementia. The risk of Alzheimer's disease has been shown to decrease with increased dietary folate intake. |
[35] |
|
1.vitamin C |
It is possible to determine the role ROS play in the inflammatory process in cAD by preventing oxidative stress in this model with the well-known antioxidant vitamin C. The role of vitamin C in the peripheral immune responses and neurodegeneration caused by colchicine-induced oxidative stress linked neuroinflammation in cAD. The neuroinflammation and neurodegeneration in the cAD hippocampus were linked to the working and reference memory deficits. When vitamin C was given to cAD (200 and 400 mg/kg BW), memory impairments improved and the hippocampus was protected from neurodegeneration and neuroinflammation. |
[39] |
|
|
2.Vitamin E |
Demonstrated that vitamin E was able to prevent A1-42 induced protein oxidation, ROS production, and neurotoxicity in primary rat embryonic hippocampal neuronal culture, possibly through the scavenging of A1-42 -induced free radicals. Vitamin E may be able to counteract the oxidative stress brought on by A. Awareness to A1-42 resulted in decreased cell viability and oxidative stress, as well as significant reductions in phospholipid and ubiquinone-10 levels, as well as in the levels of the nicotinic acetylcholine receptors' 3 and 7 subunits (nAChRs). These changes were only partially avoided by pretreatment with vitamin E. These findings suggested that nAChRs involved in the pathogenesis of AD may be expressed at lower levels and altered membrane lipid composition may be caused by A-induced lipid peroxidation |
[37] |
|
|
|
3.Vitamin D |
When it comes to diagnosing AD patients, predicting their prognosis and treatment response, and determining whether or not vitamin D is a modifiable risk factor for delaying the onset of AD, vitamin D is a reliable tool.Vitamin D promotes neurotransmission and synaptic plasticity in dopaminergic neural circuits and has anti-inflammatory and neuroprotective effects on the brain by lowering cytokine production and the burden of oxidative stress. In addition, vitamin D action in the brain has been linked to the immune cell's ability to eliminate amyloid plaques, which are a symptom of Alzheimer's disease (AD). |
[38] |
|
4.Vitamin A |
Through interactions with retinoic acid and retinoid, vitamin A and its derivatives play a significant role in the differentiation of nerve cells, the expression of neurotransmitters in the brain, and gene expression. Microglial activation is a defining aspect of nervous system inflammation. Chronic and acute neuropsychiatric diseases are characterised by inflammation. Intake of vitamin A or dietary changes may help AD patients' cognitive function. An increased risk of dementia is linked to low dietary intake of vitamin A. |
[40] |
|
|
5. |
Minerals |
When consumed in excess or inadequately, minerals also have a significant impact on the pathophysiology of Alzheimer's disease. |
[35] |
|
1.Selenium |
This disorder may be successfully prevented or treated with selenium. Because of its antioxidant properties, selenium has been suggested for treating or preventing Alzheimer's disease either alone or in combination with vitamin E. |
[43] |
|
|
2. Copper |
A number of enzymes involved in brain metabolism require copper to function properly. To maintain proper nutrient levels (homeostasis) and reduce toxic effects, sophisticated mechanisms balance copper import and export. Modified copper homeostasis is a feature of several neurodegenerative diseases, including Alzheimer's disease (AD). The pathological development of AD appears to be significantly influenced by copper homeostasis. increased oxidative stress, a significant contributor to neuronal toxicity, directly or indirectly |
[44] |
|
|
3. Iron |
Numerous crucial roles for iron are played by iron in CNS processes, such as mitochondrial energy transduction, enzyme catalysis, mitochondrial function, myelination, and the anabolism and catabolism of neurotransmitters. Increased levels of this redox-active metal in the brain due to abnormalities in iron homeostasis can lead to the metal's mislocalization and severe oxidative damage to delicate cellular and subcellular structures. Iron dyshomeostasis has been closely associated with the pathogenesis of major neurodegenerative diseases like Alzheimer's disease (AD) and others. |
[45] |
|
|
|
4. Zinc |
Normal brain activity can be modulated by zinc. We also go over how zinc affects the pathogenesis of AD as well as the production, aggregation, and degradation of the amyloid-(A) peptide. |
[46] |
|
5. Polyphenols |
In order to determine the relationship between foods high in polyphenols and amyloid accumulation and the reduction of oxidative stress in AD patients, several animal studies have been conducted. |
[47] |
|
|
6. |
Alzheimer's disease prevention and management: The role of dietary habits and Diet
|
In people with intact cognitive function, therapeutic diets may complement or even completely replace medication. Alzheimer disease can be managed by maintaining diet. |
[61] |
|
|
1. Mediterranean Diet |
The Mediterranean diet (MeDi) seems promising for preventing AD, including the earlier stages of predementia. As a matter of fact, the MeDi diet, which is based on customary eating practises in Greece, Southern Italy, and other Mediterranean regions are characterised by high consumption of fruits and vegetables, cereals, legumes, olive oil, nuts, and seeds as the main sources of fat, moderate consumption of fish, low to moderate consumption of dairy products and alcohol (wine), and low intake of red and processed meats, albeit with regional variations. Growing evidence supports the rationale that following this dietary pattern can be a preventative measure for lowering the risk of cognitive decline, mild cognitive impairment (MCI), and Alzheimer's disease (AD). This evidence points to the neuroprotective potential of the MeDi. |
[48, 49,50] |
|
2. DASH Diet |
Fruits, vegetables, nuts, whole grains, low-fat dairy products, fish, and poultry are all abundant in nutrients that lower blood pressure, such as potassium, calcium, "lean proteins," minerals, and fibre, and are included in the DASH diet. Many cardiovascular risk factors that contribute to the onset of dementia and AD, such as high blood pressure or LDL cholesterol, have been shown to be mitigated by dietary patterns, at the very least.in part by modifying the pathological processes (oxidative stress, inflammation, and insulin resistance) that characterise the physiopathology of AD |
[51] |
A. Antioxidant
Antioxidants should be helpful in the treatment of AD because oxidative processes are thought to be significant contributors to neurodegeneration. A substance known as an antioxidant interacts with free radicals to neutralise them. The phrase is frequently used to refer to inhibitors of lipid peroxidation that break chains. However, free radicals also harm proteins, DNA and almost any type of biomolecule in addition to lipid peroxidation. Numerous studies have demonstrated that oxidative stress is a precursor to AD and that antioxidants have reduced its harmful effects [52]. In AD, oxidative stress contributes to the development of plaques and tangles that harm neuronal cells. There are no medications that significantly enhance cognition or slow the progression of AD. Since diet may affect inflammation and oxidative stress in the brain, it has been recognised as a modifiable lifestyle factor that can affect the risk of developing AD. Natural antioxidants found in foods, such as polyphenols, carotenoids, and vitamins C and E, are thought to lower oxidative stress and have potential as a preventative measure or treatment for AD. In animal models of AD, several antioxidants have shown promise. The low bioavailability of antioxidants may make them less effective when used. Furthermore, because they can act as prooxidants, antioxidants that are administered in high concentrations may have negative effects. In studies with long-term exposure to substances with high bioavailability, potential effects of natural antioxidants in the prevention of AD should be evaluated [53].
B. Dietary fat
Lipids are an essential part of cell membranes and are crucial for both brain and human health. Lipid homeostasis disruption is linked to both neurologic disorders and neurodegenerative diseases like Alzheimer's disease because the brain is highly enriched in lipids (AD). Out of all the different fatty acid species, the majority of studies confirm that higher dietary intakes of saturated fatty acids (SFAs) are associated with an increased risk of AD and cognitive decline [42, 54]. According to the Chicago Health and Aging Project (CHAP), trans-fat had a 2.5 fold higher risk of causing AD than saturated fat, which had a 2.2 fold higher risk in those in the upper quintile of consumption. Transgenic mice fed a westernised diet containing 40% SFA for 4 months showed elevated cerebral a levels compared to soy oil-based diet, whereas a levels in humans with DHA-supplemented diets were lower than those with soy oil-based diet. The Mediterranean diet, which is frequently recommended due to its links to postponing age-related cognitive decline and lowering risks of AD, is a diet rich in MUFAs. However, dietary intake of MUFAs was frequently comparable to that of SFA and Trans FAs. Studies examining the effects of dietary lipids and AD in the real world are frequently muddled by the various FA compositions, which include both advantageous and detrimental FAs, yielding unfavourable results [54]. When consumed in moderation, unsaturated fats lower the risk of Alzheimer's, whereas saturated fats raise the risk of the disease in middle age [35].
C. Carbohydrates
The role of carbohydrates in amyloidosis has been known since the discovery of amyloid, our current knowledge of the part polysaccharides play in the pathogenesis of amyloid deposition in Alzheimer disease and other amyloidoses is limited to research on glycoconjugates like heparan sulphate proteglycan. In view of the decreased ability to use glucose in Alzheimer's disease, the discovery of amylose in the illness's brain, and the involvement of amyloid in the aetiology of the disease, we proposed that polysaccharides might have a wider range of functions[55] .Alzheimer's disease may occur when there is an excess of dietary carbohydrates, especially fructose, along with a relative shortage in dietary lipids and cholesterol.Strong oxidising agents like glutamate have been proven to cause oxidative stress in neurons. So, increased risk of oxidative damage is required for optimal function under conditions of low cholesterol, and at the same time, low cholesterol makes cell membranes more vulnerable to oxidative damage [56].
D. Vitamins
Vitamins are strong antioxidants. The environment in the brain becomes more and more contaminated with free radicals, which causes a steady decline in cognitive function that worsens dementia [35]. Both male and female AD patients consume less vitamin K than the healthy control group [57]. If more tocopherol forms are consumed than just a-tocopherol, vitamin E may be protective. In a significant prospective cohort study, niacin, particularly from food sources, was linked to lessening cognitive decline. Consuming B12, B6, and folate did not increase the risk of AD in either gender in relatively sizable prospective and longitudinal studies. In contrast to vitamin B6 or B12, folate levels (blood levels and intake) were lower in AD patients, according to a prospective observational cohort study. Another observational study found that those with low levels of folate or vitamin B12 had a higher risk of developing Alzheimer's disease or dementia [58]. Vitamin E and vitamin C together increased vitamin concentrations in plasma and cerebrospinal fluid and reduced the susceptibility of lipoproteins to oxidation in both males and females [59]. .Vitamin D promotes neurotransmission and synaptic plasticity in dopaminergic neural circuits and has anti-inflammatory and neuroprotective effects on the brain by lowering cytokine production and the burden of oxidative stress [38]. Chronic and acute neuropsychiatric diseases are characterised by inflammation. Intake of vitamin A or dietary changes may help AD patients' cognitive function. An increased risk of dementia is linked to low dietary intake of vitamin A [40].
E. Minerals
When consumed in excess or inadequately, minerals also have a significant impact on the pathophysiology of Alzheimer's disease. This disorder may be successfully prevented or treated with selenium. Because of its antioxidant properties, selenium has been suggested for treating or preventing Alzheimer's disease either alone or in combination with vitamin E [35,43]. In order to determine the relationship between foods high in polyphenols and amyloid accumulation and the reduction of oxidative stress in AD patients, several animal studies have been conducted [47]. A 0.87-fold increase in the risk of AD is linked to an increase in copper. Higher magnesium (Mg) is associated with AD negatively [60]. Increased levels of this redox-active metal in the brain due to abnormalities in iron homeostasis can lead to the metal's mislocalization and severe oxidative damage to delicate cellular and subcellular structures [46].
F. Dietary Habits
In people with intact cognitive function, therapeutic diets may complement or even completely replace medication. However, it is appropriate to liberalise most diet prescriptions for individuals with progressive dementias who have a decreased life expectancy. Lipid- lowering medications are typically stopped, and a low-cholesterol diet is only permitted if the serum cholesterol level is greater than 300 mg/dl. With the concurrent diagnosis of hypertension and/or foot edoema, sodium restrictions at the level of 3 to 4 g sodium, which constitute a no-added-s (NAS) diet, are continued. Poor dietary habits or modifications to a medical condition should cause a reevaluation of the recommended diet as dementia progresses [61]. Fish consumption has a protective effect against cognitive decline, which can lower the risk of dementia and Alzheimer's disease, according to epidemiological studies. Regular consumption of fruits and vegetables in a medium to high proportion may lower the risk of dementia and Alzheimer's disease. According to Morris et al. (2006), the higher consumption of fruits and vegetables and lower incidence of Alzheimer's disease may be related. Fruits and vegetables are enriched with micronutrients like antioxidants, bioactive compounds like vitamins, minerals, and polyphenols that are important to the brain, and they also have low or no saturated or trans fats. By reducing the vascular changes and structural changes in the brain that accompany cognitive decline, dairy containing vitamin D, phosphorus, and magnesium may lower the risk of cognitive impairment [35].
G. Nutritional Problem Stages
- Weight Loss
Nutritional problems are related to progressive dementias, particularly in the advanced and terminal stages. Weight loss is the most prevalent and widely used. However, losing weight is a potential issue at every point in the disease's progression. As much as six years before the actual diagnosis, AD was suspected because of weight loss.[61]
2. Hydration
A person with AD is more likely to consume less fluid than is ideal. This risk is brought on by antagonistic feeding behaviours, food refusal, communication difficulties, forgetfulness, and dependence on others for daily living activities. As patients with dementia lose the ability to communicate their thirst, it falls to the carer to make sure that they are getting enough fluids. Assessing or diagnosing chronic or mild dehydration—up to 1% loss of body weight—can be challenging.[61,62]
3. Dysphagia
Dysphagia, or difficulty swallowing, is a significant symptom of dementia. According to estimates, up to 45% of dementia patients experience swallowing difficulties to some extent. Dysphagia is frequently anticipated in the late and moderate stages of AD. Patients with mild to moderate AD may experience inappropriate eating, apathy towards food and eating, choking and aspiration symptoms, and feeding and swallowing apraxia. using electrophysiological techniques, investigate oropharyngeal swallowing in mild, moderate, and severe stages of Alzheimer's disease (AD) patients and compare them with elderly and young healthy participants who are age-matched [63].
H. Caloric Needs
The progressive nature of AD causes a tremendous fluctuation in caloric needs and necessitates a continuous process of calorie needs assessment. The degree of activity and mobility must be taken into account. Caloric expenditure rises as a result of pacing and increased agitation. There is some indication that people with AD generally need more energy to maintain their weight; [64] beneficial for estimating calorie needs. The patient's caloric needs will decrease as their level of mobility declines. It's crucial to keep an eye on changes in functional mobility to avoid unintended weight gain [61].
I. Apraxia
One of the cognitive abnormalities that distinguishes Alzheimer's disease is apraxia [65]. Apraxia can also be a symptom of parkinsonian disorders such dementia with Lewy bodies. It can sometimes be difficult to distinguish between illnesses that exhibit both parkinsonism and cognitive decline (like DLB and Alzheimer's disease), especially given that it is well known that Lewy bodies and the pathology of Alzheimer's disease (senile plaques and neurofibrillary tangles) frequently coexist. Some believe that the timing of apraxia in relation to the commencement of the disease and its combination with other symptoms (such as hallucinations and changes in consciousness level) may actually be useful in the diagnosis [66]. It has been established that self-feeding and swallowing modifications take place early on in AD. Addressing the challenge of sustaining independence demands viewing each patient as a distinct individual and making individual assessments of eating and self-feeding difficulties. Chewing problems are a sign of weakened muscles, altered oral sensation, or loss of voluntary control over the oral structure. Moreover, insufficient saliva production may make it harder for someone who has neurodegenerative alterations to swallow food since they can't transport the bolus of food from their mouth to their throat effectively. Reduced saliva production due to even slight dehydration is a related issue [67].
J. Dietary Behaviour Disorders
The nutritional status and quality of life of older persons with Alzheimer's disease are significantly impacted by dietary behaviour disorders, which are widespread in older adults and impair food consumption, nutrition status, and maintenance of body weight (AD). Dementia and cognitive decline both alter eating habits, which has significant effects on nutritional status and quality of life (especially as the disease develops, While the symptomatology of AD is renowned for its variability, the following characteristics are often constant: Early-stage disease-related memory loss impairs a person's ability to prepare and buy food, as well as all other food-related behaviours [68].
K. Stages of Nutritional Problem for Alzheimer Disease
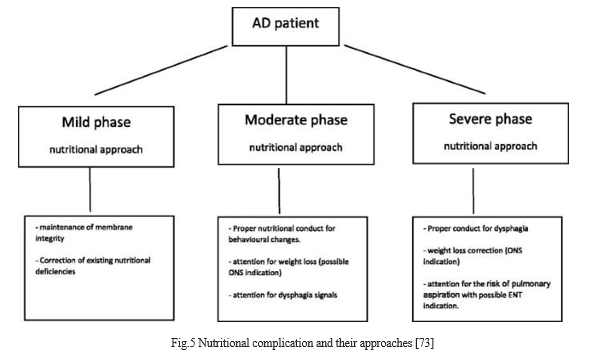
- Mildstae
At this level, the patient feeds themselves, and significant nutritional changes have not been confirmed. To assess any changes in body weight, it is advised to manage the nutritional situation. The development of our understanding of the pathophysiology of this illness has made it possible to link synaptic loss to a protective factor for damaged neurons in AD. The prevalence of dementia is lower in nations with dietary [patterns typified by high consumption of fish, fruits, and vegetables (foods that often offer higher amounts of antioxidants and polyunsaturated fatty acids) [69]. Early stages of AD, the nutritional treatment has concentrated on two crucial areas; correctly educating carers and patients on body weight reduction prevention; and reducing synapse loss [70].
2. Moderate Stage
Moderately significant behavioural alterations that affect feeding and frequently lead to an inadequate supply of nutrients for nutritional status maintenance are observed. Some of the symptoms are increased mealtime, distraction, apathy, refusal to eat, difficulty swallowing or chewing food, and difficulty chewing [71]. It is significant to note that the use of multiple medications, adopted for the treatment of AD and also their behavioural symptoms, increases the risk of negative effects on the gastrointestinal tract (vomiting, diarrhoea, nausea, vomiting, and loss of taste, smell, and appetite), which can harm food intake and also contribute to weight loss in these patients [72].
3. Severe Stage
The patient exhibits severe anatomical and physiological changes at this stage of the illness, including weakened oral and/or lingual muscles that make chewing challenging; a decrease in smell and taste that is frequently brought on by the use of medications that alter the viscosity and volume of saliva produced, reducing oral sensitivity and ultimately leading to dysphagia[69]. Main implications of dysphagia include hunger and dehydration induced by poor meals due to the alteration of food consistency. The carer frequently modifies the feeding consistency, adds extra water, and lowers the caloric density of the meal in an effort to adjust to symptoms. Any problem moving food effectively from the mouth to the stomach through the relevant steps of a complicated neurological mechanism is referred to as dysphagia [73].
Conclusion
Alzheimer\'s disease is a degenerative brain condition that is now recognised as a serious global health issue as a result of the ageing global population. Even though it is well recognised that nutrition has a substantial impact on the prevention and progression of Alzheimer\'s disease, there are currently little and disputed literature data available. For Alzheimer\'s disease, it is recommended that the reasonable eating recommendations for the aged be applied. The review provides an overview of all approaches to treating Alzheimer\'s disease, which means both allopathic and nutritional management are advised. Antioxidants, B vitamins, and polyunsaturated fatty acids are examples of nutrients in the diet that are known to be neuroprotective. According to certain research, eating regimens including the Mediterranean diet, DASH (Dietary Approaches to Control Hypertension), and MIND (Mediterranean-DASH) Diet can reduce the risk of Alzheimer\'s disease development (Intervention for Neurodegenerative Delay). Hence, maintaining a healthy nervous system may be a useful strategy in the fight against Alzheimer\'s.
References
[1] Anand, A., Patience, A.A., Sharma, N. and Khurana, N., 2017. The present and future of pharmacotherapy of Alzheimer’s disease: A comprehensive review. European journal of pharmacology, 815, pp.364-375. [2] Abubakar, M.B., Sanusi, K.O., Ugusman, A., Mohamed, W., Kamal, H., Ibrahim, N.H., Khoo, C.S. and Kumar, J., 2022. Alzheimer’s disease: an update and insights into pathophysiology. Frontiers in Aging Neuroscience, 14. [3] Sarkar, S. and Chegu Krishnamurthi, M., 2022. Nutritional, Dietary, and Lifestyle Approaches for Prevention and Management of Alzheimer’s Disease. In Role of Nutrients in Neurological Disorders (pp. 61-84). Singapore: Springer Singapore. [4] Pivi, G.A.K., Vieira, N.M.D.A., da Ponte, J.B., de Moraes, D.S.C. and Bertolucci, P.H.F., 2017. Nutritional management for Alzheimer’s disease in all stages: mild, moderate, and severe. Nutrire, 42, pp.1-6. [5] Castellani, R.J., Rolston, R.K. and Smith, M.A., 2010. Alzheimer disease. Disease-a-month: DM, 56(9), p.484. [6] Menghani, Y.R., Bhattad, D.M., Chandak, K.K., Taksande, J.B. and Umekar, M.J., 2021. A Review: Pharmacological and herbal remedies in The Management of Neurodegenerative disorder (Alzheimer’s). International Journal of Pharmacognosy and Life Science, 2(1), pp.18-27. [7] Bush, A.I., 2003. The metallobiology of Alzheimer\'s disease. Trends in neurosciences, 26(4), pp.207-214. [8] Jin, J., 2015. Alzheimer disease. Jama, 313(14), pp.1488-1488. [9] Povova, J., Ambroz, P., Bar, M., Pavukova, V., Sery, O., Tomaskova, H. and Janout, V., 2012. Epidemiological of and risk factors for Alzheimer’s disease: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 156(2), pp.108-14. [10] Liu, P.P., Xie, Y., Meng, X.Y. and Kang, J.S., 2019. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal transduction and targeted therapy, 4(1), p.29. [11] Kukull, W.A., 2001. The association between smoking and Alzheimer’s disease: effects of study design and bias. Biological psychiatry, 49(3), pp.194-199. [12] Huang, W.J., Zhang, X. and Chen, W.W., 2016. Association between alcohol and Alzheimer\'s disease. Experimental and therapeutic medicine, 12(3), pp.1247-1250. [13] Picone, P., Di Carlo, M. and Nuzzo, D., 2020. Obesity and Alzheimer’s disease: Molecular bases. European Journal of Neuroscience, 52(8), pp.3944-3950. [14] Wilson, R.S., Scherr, P.A., Schneider, J.A., Tang, Y. and Bennett, D.A., 2007. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology, 69(20), pp.1911-1920. [15] Lim, S.Y., Kim, E.J., Kim, A., Lee, H.J., Choi, H.J. and Yang, S.J., 2016. Nutritional factors affecting mental health. Clinical Nutrition Research, 5(3), pp.143-152. [16] Silva, M.V.F., Loures, C.D.M.G., Alves, L.C.V., de Souza, L.C., Borges, K.B.G. and Carvalho, M.D.G., 2019. Alzheimer’s disease: risk factors and potentially protective measures. Journal of biomedical science, 26, pp.1-11. [17] Santos, Cláudia Y., Peter J. Snyder, Wen-Chih Wu, Mia Zhang, Ana Echeverria, and Jessica Alber. \"Pathophysiologic relationship between Alzheimer\'s disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis.\" Alzheimer\'s & Dementia: Diagnosis, Assessment & Disease Monitoring 7 (2017): 69-87. [18] Gabin, J.M., Tambs, K., Saltvedt, I., Sund, E. and Holmen, J., 2017. Association between blood pressure and Alzheimer disease measured up to 27 years prior to diagnosis: the HUNT Study. Alzheimer\'s research & therapy, 9, pp.1-12. [19] Lattanzi, S., Luzzi, S., Provinciali, L. and Silvestrini, M., 2015. Blood pressure variability in Alzheimer\'s disease and frontotemporal dementia: the effect on the rate of cognitive decline. Journal of Alzheimer\'s Disease, 45(2), pp.387-394. [20] Biddle, K.D., Uquillas, F.D.O., Jacobs, H.I., Zide, B., Kirn, D.R., Rentz, D.M., Johnson, K.A., Sperling, R.A. and Donovan, N.J., 2019. Social engagement and amyloid-?-related cognitive decline in cognitively normal older adults. The American Journal of Geriatric Psychiatry, 27(11), pp.1247-1256. [21] Meeker, K.L., Wisch, J.K., Hudson, D., Coble, D., Xiong, C., Babulal, G.M., Gordon, B.A., Schindler, S.E., Cruchaga, C., Flores, S. and Dincer, A., 2021. Socioeconomic status mediates racial differences seen using the AT (N) framework. Annals of neurology, 89(2), pp.254-265. [22] Lyketsos, C.G. and Olin, J., 2002. Depression in Alzheimer’s disease: overview and treatment. Biological psychiatry, 52(3), pp.243-252. [23] Herrmann, N., Ruthirakuhan, M., Gallagher, D., Verhoeff, N.P.L., Kiss, A., Black, S.E. and Lanctôt, K.L., 2019. Randomized placebo- controlled trial of nabilone for agitation in Alzheimer\'s disease. The American Journal of Geriatric Psychiatry, 27(11), pp.1161-1173. [24] Schneider, L.S. and Dagerman, K.S., 2004. Psychosis of Alzheimer\'s disease: clinical characteristics and history. Journal of psychiatric research, 38(1), pp.105-111. [25] Boccardi, V., Ruggiero, C., Patriti, A. and Marano, L., 2016. Diagnostic assessment and management of dysphagia in patients with Alzheimer’s disease. Journal of Alzheimer\'s Disease, 50(4), pp.947-955. [26] Manabe, T., Mizukami, K., Akatsu, H., Teramoto, S., Yamaoka, K., Nakamura, S., Ohkubo, T., Kudo, K. and Hizawa, N., 2016. Influence of pneumonia complications on the prognosis of patients with autopsy?confirmed A lzheimer\'s disease, dementia with L ewy bodies, and vascular dementia. Psychogeriatrics, 16(5), pp.305-314. [27] Duyckaerts, C., Delatour, B. and Potier, M.C., 2009. Classification and basic pathology of Alzheimer disease. Acta neuropathologica, 118, pp.5-36. [28] Kumar, A. and Singh, A., 2015. A review on Alzheimer\'s disease pathophysiology and its management: an update. Pharmacological reports, 67(2), pp.195-203. [29] Dal Prà 1, Chiarini A, Gui L, Chakravarthy B, Pacchiana R, Gardenal E, et al. Do astrocytes collaborate with neurons in spreading the \"infectious\" AB and tau drivers of Alzheimer\'s disease? Neuroscientist 2014. 1073858414529828. [30] Dwomoh, L., Tejeda, G.S. and Tobin, A.B., 2022. Targeting the M1 muscarinic acetylcholine receptor in Alzheimer’s disease. Neuronal Signaling, 6(1), p.NS20210004. [31] Cummings, J.L., Schneider, E., Tariot, P.N. and Graham, S.M., 2006. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology, 67(1), pp.57-63. [32] Sato, T., Hanyu, H., Hirao, K., Kanetaka, H., Sakurai, H. and Iwamoto, T., 2011. Efficacy of PPAR-? agonist pioglitazone in mild Alzheimer disease. Neurobiology of aging, 32(9), pp.1626-1633. [33] Wallach, J., Colestock, T. and Adejare, A., 2017. Receptor targets in Alzheimer’s disease drug discovery. In Drug Discovery Approaches for the Treatment of Neurodegenerative Disorders (pp. 83-107). Academic Press [34] Imbimbo, B.P. and Peretto, I., 2009. Semagacestat, a gamma-secretase inhibitor for the potential treatment of Alzheimer’s disease. Curr Opin Investig Drugs, 10(7), pp.721-30. [35] Sarkar, S. and Chegu Krishnamurthi, M., 2022. Nutritional, Dietary, and Lifestyle Approaches for Prevention and Management of Alzheimer’s Disease. In Role of Nutrients in Neurological Disorders (pp. 61-84). Singapore: Springer Singapore. [36] Chang, Y.T., Chang, W.N., Tsai, N.W., Huang, C.C., Kung, C.T., Su, Y.J., Lin, W.C., Cheng, B.C., Su, C.M., Chiang, Y.F. and Lu, C.H., 2014. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer’s disease: a systematic review. BioMed research international, 2014. [37] Gugliandolo, A., Bramanti, P. and Mazzon, E., 2017. Role of vitamin E in the treatment of Alzheimer’s disease: Evidence from animal models. International Journal of Molecular Sciences, 18(12), p.2504. [38] Bivona, G., Lo Sasso, B., Gambino, C.M., Giglio, R.V., Scazzone, C., Agnello, L. and Ciaccio, M., 2021. The role of vitamin D as a biomarker in alzheimer’s disease. Brain sciences, 11(3), p.334. [39] Sil, S., Ghosh, T., Gupta, P., Ghosh, R., Kabir, S.N. and Roy, A., 2016. Dual role of vitamin C on the neuroinflammation mediated neurodegeneration and memory impairments in colchicine induced rat model of Alzheimer disease. Journal of Molecular Neuroscience, 60, pp.421-435. [40] Mielech, A., Pu?cion-Jakubik, A., Markiewicz-?ukowska, R. and Socha, K., 2020. Vitamins in Alzheimer’s disease—Review of the latest reports. Nutrients, 12(11), p.3458. [41] Henderson, S.T., 2004. High carbohydrate diets and Alzheimer\'s disease. Medical hypotheses, 62(5), pp.689-700. [42] Shah, R., 2013. The role of nutrition and diet in Alzheimer disease: a systematic review. Journal of the American Medical Directors Association, 14(6), pp.398-402. [43] Barchielli, G., Capperucci, A. and Tanini, D., 2022. The role of selenium in pathologies: An updated review. Antioxidants, 11(2), p.251. [44] Donnelly, P.S., Xiao, Z. and Wedd, A.G., 2007. Copper and Alzheimer\'s disease. Current opinion in chemical biology, 11(2), pp.128- 133. [45] Lane, D.J., Ayton, S. and Bush, A.I., 2018. Iron and Alzheimer’s disease: an update on emerging mechanisms. Journal of Alzheimer\'s Disease, 64(s1), pp.S379-S395 [46] Watt, N.T., Whitehouse, I.J. and Hooper, N.M., 2011. The role of zinc in Alzheimer\'s disease. International Journal of Alzheimer’s disease, 2011. [47] Colizzi, C., 2019. The protective effects of polyphenols on Alzheimer\'s disease: a systematic review. Alzheimer\'s & Dementia: Translational Research & Clinical Interventions, 5, pp.184-196. [48] Knight, A., Bryan, J. and Murphy, K., 2016. Is the Mediterranean diet a feasible approach to preserving cognitive function and reducing risk of dementia for older adults in Western countries? New insights and future directions. Ageing Research Reviews, 25, pp.85-101. [49] Solfrizzi, V., Frisardi, V., Seripa, D., Logroscino, G., P Imbimbo, B., D\'Onofrio, G., Addante, F., Sancarlo, D., Cascavilla, L., Pilotto, A. and Panza, F., 2011. Mediterranean diet in predementia and dementia syndromes. Current Alzheimer Research, 8(5), pp.520-542. [50] Solfrizzi, V. and Panza, F., 2014. Mediterranean diet and cognitive decline. A lesson from the whole-diet approach: what challenges lie ahead?. Journal of Alzheimer\'s Disease, 39(2), pp.283-286 [51] Cremonini, A.L., Caffa, I., Cea, M., Nencioni, A., Odetti, P. and Monacelli, F., 2019. Nutrients in the Prevention of Alzheimer’s Disease. Oxidative medicine and cellular longevity, 2019. [52] Veurink, G., Perry, G. and Singh, S.K., 2020. Role of antioxidants and a nutrient rich diet in Alzheimer\'s disease. Open Biology, 10(6), p.200084. [53] Lange, K.W., Lange, K.M., Nakamura, Y. and Li, S., 2020. Do natural antioxidants play a role in Alzheimer’s disease?. Journal of Food Bioactives, 11. [54] Kao, Y.C., Ho, P.C., Tu, Y.K., Jou, I.M. and Tsai, K.J., 2020. Lipids and Alzheimer’s disease. International journal of molecular sciences, 21(4), p.1505. [55] Castellani, R.J., Perry, G. and Smith, M.A., 2007. The role of novel chitin-like polysaccharides in Alzheimer disease. Neurotoxicity research, 12, pp.269-274. [56] Seneff, S., Wainwright, G. and Mascitelli, L., 2011. Nutrition and Alzheimer\'s disease: the detrimental role of a high carbohydrate diet. European Journal of Internal Medicine, 22(2), pp.134-140. [57] Presse, N., Shatenstein, B., Kergoat, M.J. and Ferland, G., 2008. Low vitamin K intakes in community-dwelling elders at an early stage of Alzheimer\'s disease. Journal of the American Dietetic Association, 108(12), pp.2095-2099. [58] Shah, R., 2013. The role of nutrition and diet in Alzheimer disease: a systematic review. Journal of the American Medical Directors Association, 14(6), pp.398-402. [59] Kontush, A., Mann, U., Arlt, S., Ujeyl, A., Lührs, C., Müller-Thomsen, T. and Beisiegel, U., 2001. Influence of vitamin E and C supplementation on lipoprotein oxidation in patients with Alzheimer’s disease. Free Radical Biology and Medicine, 31(3), pp.345-354. [60] Cheng, W.W., Zhu, Q. and Zhang, H.Y., 2019. Mineral nutrition and the risk of chronic diseases: a Mendelian randomization study. Nutrients, 11(2), p.378. [61] Morris, J. and Volicer, L., 2001. Nutritional management of individuals with Alzheimer\'s disease and other progressive dementias. Nutrition in Clinical Care, 4(3), pp.148-155. [62] Schoeller, D.A., 1989. Changes in total body water with age. The American journal of clinical nutrition, 50(5), pp.1176-1181. [63] Seçil, Y., Ar?c?, ?., ?ncesu, T.K., Gürgör, N., Beckmann, Y. and Ertekin, C., 2016. Dysphagia in Alzheimer\'s disease. Neurophysiologie Clinique/Clinical Neurophysiology, 46(3), pp.171-178. [64] Spindler, A.A., Renvall, M.J., Nichols, J.F. and Ramsdell, J.W., 1996. Nutritional status of patients with Alzheimer\'s disease: a 1-year study. Journal of the American Dietetic Association, 96(10), pp.1013-1018 [65] Lesourd, M., Le Gall, D., Baumard, J., Croisile, B., Jarry, C. and Osiurak, F., 2013. Apraxia and Alzheimer’s disease: review and perspectives. Neuropsychology review, 23, pp.234-256. [66] Zadikoff, C. and Lang, A.E., 2005. Apraxia in movement disorders. Brain, 128(7), pp.1480-1497. [67] Kleiner, S.M., 1999. Water: an essential but overlooked nutrient. Journal of the American Dietetic Association, 99(2), pp.200-206 [68] Shatenstein, B., Kergoat, M.J., Reid, I. and Chicoine, M.E., 2008. Dietary intervention in older adults with early-stage Alzheimer dementia: early lessons learned. The Journal of Nutrition Health and Aging, 12, pp.461-469. [69] Féart, C., Samieri, C., Rondeau, V., Amieva, H., Portet, F., Dartigues, J.F., Scarmeas, N. and Barberger-Gateau, P., 2009. Adherence to a Mediterranean Diet, Cognitive Decline, and Risk of Dementia (vol 302, pg 638, 2009). JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION, 302(22), pp.2436-2436. [70] Pivi, G.A.K., Kato, L.C. and França, A.P., 2015. Condutas dietéticas para idoso com doenças no Sistema Neurocognitivo. Tratado de Nutrição em Gerontologia. 1st ed. Baruer: Manole, pp.231-7. [71] Correia, S.D.M., 2010. Avaliação fonoaudiológica da deglutição na doença de Alzheimer em fases avançadas (Doctoral dissertation, Universidade de São Paulo). [72] Appolinario, J.C. and Bacaltchuk, J., 2002. Tratamento farmacológico dos transtornos alimentares. Brazilian Journal of Psychiatry, 24, pp.54-59. [73] Pivi, G.A.K., Vieira, N.M.D.A., da Ponte, J.B., de Moraes, D.S.C. and Bertolucci, P.H.F., 2017. Nutritional management for Alzheimer’s disease in all stages: mild, moderate, and severe. Nutrire, 42, pp.1-6.
Copyright
Copyright © 2024 Ms. Sonal R. Barde, Dr. Deepa S. Mandlik, Samina B. Sanadi, Ms. Pranali Bangaiya, Dr. Sangan Kanthale. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63797
Publish Date : 2024-07-29
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

