Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Optimization of Three Components, One Pot Synthesis of Aminobenzylnaphthol Exploiting Electrophilicity of Azomethines under Varying Conditions
Authors: Sushma Rani, M. K. Singh
DOI Link: https://doi.org/10.22214/ijraset.2025.66779
Certificate: View Certificate
Abstract
The synthesis of aminobenzylnaphthols by organocatalysis has generated interest due to the mild reaction conditions and environmental benefits. It has been shown that (1,4-diazacyclo[2.2.2]octane) has demonstrated high efficiency as an organocatalyst for the three component condensation reaction. These reactions lead to the synthesis of a novel class of aminobenzylnaphthols under various solvent conditions offering remarkable advantages like: mild reaction conditions, high yields, selectivity and simplicity. This protocol is particularly appealing for the synthesis of complex organic molecules with potential applications in pharmaceuticals and material science. Overall, the use of organocatalysts in aminobenzylnaphthol synthesis presents a versatile approach with potential for the further development maintaining the guidelines of greener chemistry.
Introduction
I. INTRODUCTION
Compounds containing 1,3-aminooxygenated functional groups are indeed significant in medicinal chemistry and natural products [1].These structures often contribute to biological activity due to their ability to participate in hydrogen bonding and interaction with biological targets. The design of synthetic drugs [1-3] often incorporates these functional groups to enhance efficacy and optimize pharmacokinetic properties. Aminonaphthols, commonly referred to as Betti bases [4],are an important class of compounds characterized by a naphthol moiety with an amino group. Aminonaphthols or Betti bases, exhibit a range of beneficial biological properties, including analgesic, antibacterial, hypotensive and bardycardiacactivities[5-8] fig1. The diverse biological activities of aminonaphthols highlight their importance in medicinal chemistry and the potential for further exploration in drug development[4,9].
Since Betti’s initial report in 1900, numerous methods have been developed for the synthesis of aminonaphthols. The preparation of aminonaphthols typically involves(scheme 1) the reaction of 2-naphthol with an aldehyde and aliphatic amine[4] through a process known as an imine. In this reaction, the aldehyde reacts with the amine to form an intermediate imine, which subsequently undergoes nucleophilic attack by the 2-naphthol. Hydroysis of performed amidoalkylnaphthols is another effective approach to synthesize aminonaphthols [10-19]. The synthesis of amidoalkylnaphthols has generated significant interest due to their versatile applications in various fields, including pharmaceuticals, agrochemicals and material science. These compounds often exhibit biological activities, making them valuable in drug development. Many traditional methods for synthesizing amidoalkylnaphthols often come with challenges including: low product yields, expensive catalysts, long reaction times and laborious workup procedures[4-9]. These disadvantages have prompted researchers to seek more efficient, cost effective methods, such as greener solvents, alternative catalysts, or one pot reactions. The synthesis of arylaminonaphthols after presents[23-24] additional challenges[20-22] compared to aliphatic counter parts. The synthesis of hetero arylaminonaphthols using electron rich heteroaromatic amines as amine sources[25-27] .Organocatalysts have indeed demonstrated significant potential in multi-component reaction (MCRs)[28] due to high efficiency and selectivity.
The advantages of using organic catalysts can be: easy preparation or availabilities, milder reaction conditions, selectivity and compatibility with numerous functional groups [29]. Recent studies have highlighted the effectiveness of organocatalysts, particularly derivatives of N,N-dialkylethanolamine, in catalyzing Micheal addition [30] and friedel-crafts alkylation reactions[31,32].
To the best of our knowledge, there have been reported studies on the synthesis of Betti bases using organocatalysts. Betti bases, which are significant in various applications including medicinal chemistry , typically involve multi-component reaction. Exploring organocatalysts for this synthesis could offer new pathways for efficient and selective production, potentially leveraging their advantages in milder conditions and broad functional group compatibility.
The use of bifunctional organocatalysts for synthesizing alkyl and arylnaphthol derivatives through a one-pot three component[33-37] condensation is intriguing. This method effectively combines β-naphthol, aldehydes, and arylamines, potentially leading to efficient synthesis pathways with minimal byproducts. The bifunctional nature of the organocatalysts likely enhances reactivity and selectivity, streamlining the process. Additionally, characterizing the final products using techniques like NMR or mass spectroscopy would be beneficial for confirming the success of the synthesis.
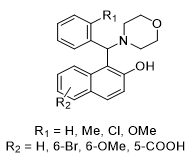
Fig 1: An aminonaphthols with antipatin and antibacterial activities
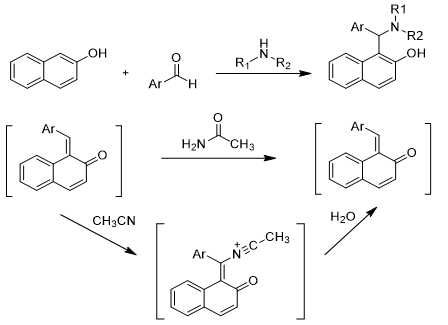
Scheme 1: Methods for synthesis of aminonaphthols
II. EXPERIMENTAL
All chemicals were purchased from Merck or Aldrich chemical companies and were used without further purification . This ensured convenience and efficiency in the experimental procedures, maintaining the integrity of the synthesized products while minimizing the handling and processing time.
Melting points were determined using a MEL-TEMP model 1202D apparatus and are uncorrected. FTIR spectra were recorded using a Bruker Tensor 27 spectrometre in the form of KBr discs.
13C NMR spectra were recorded on the instrument at a frequency of 100 MHz . Chemical shifts were reported with respect to solvent signals as internal standards and coupling constant (J) are given in Hz . Multiplicities are indicated as the following: s,singlet ; d, doublet ; t,triplet; q,quartet ; m,multiplet; dd,doubleddoublet. Preparative layer chromatography was prepared using silica gel (Merk Kieselgel 60 Hf254,no. 7739)
III. RESULT AND DISCUSSION
Our studies started with the reaction of 2- naphthol (2a) with benzaldehyde(13a) and aniline (14a) as our model reaction to investigate. A range of organocatalysts was evaluated under different conditions, using different organic solvents. The results are summarized in table 1.
As given in table 1, different polar organic solvents such as ethanol(EtOH), chloroform(CHCl3) and dichloromethane(CH2Cl2) resulted in low to moderate yields at room temperature(15-45%, entries 1-12). The best results were achieved under non-polar benzene solvent, demonstrating significantly higher yields. Organocatalyst II gave a promising outcomes (70%, entry 14), other organocatalysts I & III produced lower yields (30-35%, entries 13-15) and organocatalys (IV-VI) provided poor results (50-52%, entries 16-18).
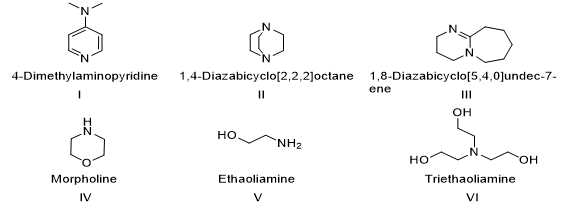
Fig 2: Organocatalysts I-VI
Since the reactions as well as products are air stable, there was no need to carry out the reactions in an inert atmosphere, which simpliflied the experimental set up and reduced the overall complexity of the process. This characteristic of the solvent benzene makes it suitable for broader applications in this type of organic synthesis. To optimize the reaction conditions, the model reaction was exposed to different amounts of organocatalyst II (5.7,8,10 &15 mol%) at different temperature using benzene solvent. (table 2)
Perusal of suggests in table 2, the best results were obtained when the reaction was performed using benzene as a solvent and 8 mol% of DABCO as the organocatalyst at 75? C. To investigate the scope and generality of this procedure, the condensation reactions of 2-naphthols with a range of aromatic aldehydes and aromatic amines was conducted in the presence of benzene solvent and DABCO as a catalyst (fig2) at 85? C ( scheme 3,Table 3). As Shown in table 2, the reactions were carried out efficiently within 30 to 45 minutes, resulting in the production of the desired products in good to excellent yields. This demonstrates the effectiveness of the DABCO catalysed condensation process under solvent . Highlighting its potential for rapid and efficient organic synthesis.(fig3)
Different substituted benzaldehydes successfully reacted with aniline and 2-naphthol , yielding the corresponding products( 15a-p) in 80-88% yields.
The reactions demonstrated goods functional group tolerance, indicating the versatility of the DABCO catalysed process. This further confirms the methods applicability for a wide range of substrates in organic synthesis.
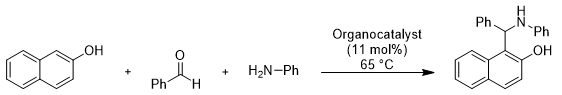
Scheme 2: synthesis of Betti bases
Table 1: Optimization of organocatalytic synthesis of arylaminonaphthol under various conditions.
|
Entry |
Catalyst (11 mol%) |
Solvent |
Time(h) |
Yield(%)a |
|
1b |
I |
CHCl3 |
24 |
20 |
|
2b |
I |
CH2Cl2 |
26 |
15 |
|
3b |
I |
EtOH |
20 |
25 |
|
4b |
II |
CHCl3 |
24 |
45 |
|
5b |
II |
CH2Cl2 |
24 |
45 |
|
6b |
II |
EtOH |
26 |
45 |
|
7b |
III |
CHCl3 |
24 |
20 |
|
8b |
IV |
CHCl3 |
24 |
25 |
|
9b |
V |
CHCl3 |
26 |
35 |
|
10b |
VI |
CHCl3 |
24 |
40 |
|
11b |
VI |
CH2Cl2 |
24 |
35 |
|
12b |
VI |
EtOH |
24 |
40 |
|
13c |
I |
C6H6 |
2 |
30 |
|
14c |
II |
C6H6 |
2 |
70 |
|
15c |
III |
C6H6 |
2 |
35 |
|
16c |
IV |
C6H6 |
2 |
50 |
|
17c |
V |
C6H6 |
2 |
52 |
|
18c |
VI |
C6H6 |
2 |
55 |
- Isolated yield
- Reaction was carried out at rt using various solvents
- Reaction carried out at 65?C under solvent benzene
Table 2 Optimization of temperature and amount of DABCO in the model reaction under solvent benzene
|
Entry |
Catalyst amount (mol%) |
Temp(?C) |
Time(min) |
Yield(%) |
|
1 |
10 |
45 |
55 |
50 |
|
2 |
10 |
40 |
45 |
58 |
|
3 |
15 |
65 |
40 |
75 |
|
4 |
15 |
60 |
45 |
70 |
|
5 |
5.7 |
55 |
45 |
61 |
|
6 |
8 |
75 |
40 |
80 |
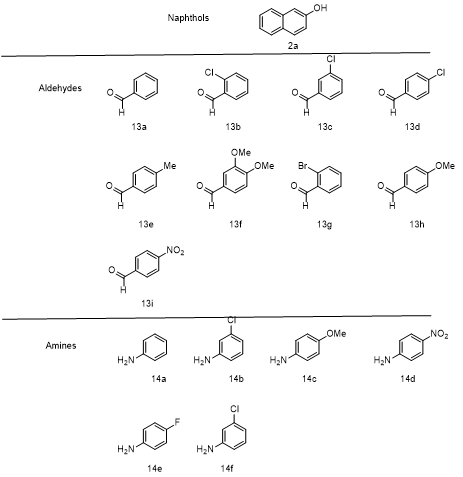
Fig 2: Diversity of reactants used

Scheme 3: Synthesis of Betti bases
Table 3 Synthesis of arylaminonaphthols using DABCO under solvent benzene
|
Entry |
Reactants |
Products |
Time(min) |
Yield(%)a |
|
1 |
2a/13a/14a |
15a |
40 |
80 |
|
2 |
2a/13b/14a |
15b |
45 |
88 |
|
3 |
2a/13c/14a |
15c |
45 |
85 |
|
4 |
2a/13d/14a |
15d |
40 |
82 |
|
5 |
2a/13e/14a |
15e |
45 |
85 |
|
6 |
2a/13f/14a |
15f |
35 |
80 |
|
7 |
2a/13g/14a |
15g |
40 |
81 |
|
8 |
2a/13h/14a |
15h |
45 |
87 |
|
9 |
2a/13a/14a |
15i |
40 |
85 |
|
10 |
2a/13a/14c |
15j |
45 |
75 |
|
11 |
2a/13a/14d |
15k |
38 |
88 |
|
12 |
2a/13a/14e |
15l |
40 |
85 |
|
13 |
2a/13a/14f |
15m |
35 |
87 |
|
14 |
2a/13d/14b |
15n |
45 |
86 |
|
15 |
2a/13e/14c |
15o |
40 |
85 |
|
16 |
2a/13d/14e |
15p |
45 |
88 |
Reaction conditions 2-naphthol/aldehyde/amines=1:1:1.5 and DABCO (8mol%) at 75?C under solvent benzene.
General procedure for the synthesis of aminonaphthols
A mixture of 2- naphthol(1mmol), aldehyde(1mmol) and an amine(1.5 mmol) was treated with DABCO Catalytic additive. The mixture was stirred at 55? C under solvent benzene in an oil bath for an appropriate duration. The progress of the reaction was monitored by TLC(acetone/chloroform/hexane: 1/2/5).Upon completion of the reaction, the mixture was allowed to cool at room temperature. The solid was collected through the filtration process. Crude products (15k, 15l, 15p) were purified by layer chromatography on silica gel using (EtOAc/n-hexane). Compounds (15a-j,15m,15n,15o) were purified by recrystallisation (EtOH/acetone). All compounds were characterized using the following analytical techniques like melting point(mp),IR,1H NMR,13C NMR and elemental (C,H,N)analysis.
1-[Phenyl(phenylamino)methyl]naphthalen-2-ol 15a
White solid ; Yield 80% ; mp : 130-135 ? C ; 1 H NMR (400MHz, CDCL3) : δ 4.21(1H, bs, N-H , disappeared in the presence of D2O), 6.23 (1H, s, methine-H), 6.86 (2H, d, J=7.8 Hz , Ar-H) ν 7.22-7.53 (10H, m, Ar-H), 7.86-7.92 (3H, m, Ar-H), 11.64 (1H, bs, OH, disappeared in the presence of D2O)ppm; 13C NMR (100 MHz, CDCl3): δ 62.4, 115.4, 117.5, 118.4, 121.8,121.9, 122.9, 125.8, 125.9, 127.2, 127.7, 127.9, 128.3, 128.5, 128.7, 128.9, 139.9, 145.7, 157.8ppm
1-[(Chlorophenyl)(phenylamino)methyl]naphthalen-2-ol 15b
Colourless crystals; Yield 88% ; mp: 155-158 ?C; FT-IR (KBr) ν 3518, 3401, 3159, 3006, 1704 ,1590 ,1329 ,854 cm-1 ; 1H NMR (400 MHz, CDCl3): δ 4.11 (1H, bs, N-H), 6.67 (1H, s, methine-H), 6.90 (2H, d, J=8Hz , Ar-H), 7.03 (1H, t, J=7.6Hz, Ar-H), 7.15-7.28 (5H, m, Ar-H), 7.33-7.48 (3H, m, Ar-H), 7.87-7.99 (2H, m, Ar-H), 11.74 (1H, bs, OH)ppm; 13C NMR (100MHz, CDCl3): δ 58.6, 111.9, 115.7, 118.9, 120.5, 121.4, 121.9, 126.5, 126.8, 127.9, 128.6, 128.9, 129.5, 129.7, 130.7, 132.9, 136.5, 145.11, 156.2 ppm. Anal. Calcd. For C23H18ClNO: C, 76.88 ; H, 5.06; N,3.99 ;Found :C, 76.61; H,5.15; N,3.87%
1-[(3-Chlorophenyl)(phenyamino)methyl]naphthalene-2-ol 15c
Colourless Crystals; Yield 85% ; mp: 148-150? C ; FT-IR (KBr) ν 3388, 3347, 3064, 2905, 1604, 1498, 1241, 764 cm-1 ;1H NMR (400MHz, CDCl3): δ 4.14 (1H, bs, N-H), 6.14 (1H, s, methane-H),6.84 (2H, d, J=8.2Hz, Ar-H), 6.95 (1H, t, J=7.5Hz, Ar-H), 7.15-7.22 (4H, m, Ar-H), 7.41 (1H, t, J=7.5Hz, Ar-H), 7.47-7.53 (3H, m, Ar-H), 7.74 (1H, s, Ar-H), 7.83-7.89 (3H, m, Ar-H), 11.46 (1H, bs, OH) ppm; 13C NMR (100MHz, CDCl3): δ 60.9, 112.3, 115.5, 119.2,120.2, 120.9, 121.9, 122.4, 125.6, 125.9, 128.1, 128.6, 129.3, 129.9, 130.5, 130.8, 142.2, 145.5, 155.5 ppm. Anal. Calcd. For C23H18ClNO: C,76.88; H,5.9; N,3.99; Found: C,76.58; H,5.16; N,3.88%
1-[(4-Chlorophenyl)(phenylamino)methyl]naphtholen-2-ol 5d
Colourless Crystals; Yield 82%; mp: 123-125? C; FT-IR (KBr) ν 3422, 3358, 3064, 2965, 1624, 1599, 1491, 1240, 764 cm-1 ;1H NMR (400MHz, CDCl3): δ 4.14 (1H, bs, N-H), 6.21 (1H, s, methane-H), 6.84 (1H, d, J=8.2Hz, Ar-H), 6.90-6.98 (1H, m, Ar-H), 7.10 (4H, t, J=8Hz, Ar-H), 7.21-7.29 (3H, m, Ar-H), 7.37-7.54 (7H, m, Ar-H), 7.83-7.89 (2H, m, Ar-H), 11.46 (1H, bs, OH)ppm; 13C NMR (100MHz, CDCl3): δ 60.11, 112.3, 115.5, 119.2, 121.8, 122.9, 123.9, 124.4, 125.6, 127.5, 127.9, 128.6, 128.8, 129.9, 132.7, 140.8, 145.5, 151.5, 155.5 ppm. Anal. Calcd. For C23H18ClNO: C,76.88; H,5.9; N,3.99; Found: C,76.48; H,5.10; N,3.89%
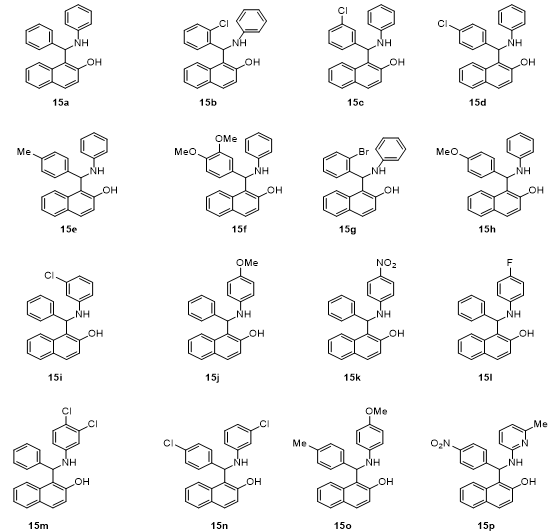
Fig 4 : Products 15a-15p
1-[(phenylamino)(p-tolyl)methyl]naphtholen-2-ol 5e
Colourless Crystals; Yield 85% ; mp: 133-135?C ; FT-IR (KBr) ν 3422, 3352, 3064, 2975, 1604, 1599, 1491, 1240 cm-1 ;1H NMR (400 MHz, CDCl3): δ 2.39 (3H, s, -CH3), 4.21 (1H, bs, N-H), 6.14 (1H, s, methane-H), 6.78 (2H, d, J=8.2Hz, Ar-H), 6.99 (1H, t, J=7.8Hz, Ar-H), 7.13-7.19 (5H ,m, Ar-H), 7.27-7.38 (4H, m, Ar-H), 7.83-7.89 (3H, m, Ar-H), 11.66 (1H, bs, OH) ppm; 13C NMR (100MHz, CDCl3): δ 20.12, 61.5, 112.9, 115.4, 118.9, 120.7, 120.9, 121.9, 125.8, 126.8, 127.9, 128.0, 128.4, 128.6, 128.9, 130.7, 137.8, 145.8, 155.5 ppm. Anal. Calcd. For C24H21NO: C,84.98; H,6.9; N,4.29; Found:C,86.68; H,6.41; N,4.09%
1-[(3,4-Dimethoxyphenyl)(phenylamino)methyl]naphtholen-2-ol 5f
Light brown solid; Yield 80% ; mp: 140-142? C ; FT-IR (KBr) ν 3399, 3318, 3019, 2965, 1599, 1511, 1240, 1028 cm-1 ;1 H NMR (400 MHz, CDCl3): δ 3.89 (3H, s, -OCH3), 3.85 (3H, s,-OCH3), 4.21 (1H, bs, N-H), 6.14 (1H, s, methine-H), 6.85-6.89 (3H, m, Ar-H), 6.92 (1H, t, J=7.5Hz, Ar-H), 6.99-7.04 (2H, m, Ar-H), 7.13-7.18 (3H, m, Ar-H), 7.31 (1H, t, J=7.5Hz, Ar-H) 7.41 (1H, m, Ar-H),7.75-7.85 (3H, m, Ar-H), 11.58 (1H, bs, OH) ppm; 13C NMR (100MHz, CDCl3): δ 54.11, 61.8, 109.8, 110.6, 113.4, 115.5, 118.9, 119.5, 120.4, 120.9, 121.8, 125.7, 127.9, 128.2, 128.6, 128.8, 130.8, 132.7, 145.8, 148.5, 148.8, 155.5 ppm. Anal. Calcd. For C25H23 NO3: C,77.98; H,6.03; N,3.69; Found: C,77.68; H,6.10; N,3.68%
1-[(2-Bromophenyl)(phenylamino)methyl]naphtholen-2-ol 5g
Light brown solid; Yield 81% ; mp: 152-154?C ; FT-IR (KBr) ν 3429, 3348, 3059, 3019, 2970,1624, 1604, 1499, 1240, 698 cm-1 ;1H NMR (400 MHz, CDCl3): δ 4.08 (1H, bs, N-H), 6.48 (1H, s, methane-H), 6.81 (2H, d, J=8.0Hz, Ar-H), 6.94 (1H, t, J=8.1Hz, Ar-H), 7.15-7.18 (6H, m, Ar-H), 7.29-7.38 (2H, t, m, Ar-H), 7.60 (1H, d, J=8.5Hz, Ar-H), 7.67-7.69 (1H, m, Ar-H), 7.79 (2H, d, J=8.8Hz, Ar-H), 11.68 (1H, bs, OH)ppm; 13C NMR (100 MHz, CDCl3 ): δ 60.8, 111.9, 115.6, 118.9, 120.5, 121.5, 121.9, 123.6, 126.2, 127.5, 127.5, 127.9, 128.2, 128.6, 129.5, 129.8, 130.5, 132.7, 138.2, 145.9, 155.9 ppm. Anal. Calcd. For C23H18 BrNO: C,69.38; H,4.53; N,3.56; Found: C,68.08; H,4.55; N,3.58%
1-[(4-Methoxyphenyl)(phenylamino)methyl]naphtholen-2-ol 5h
Light brown solid; Yield 87% ; mp: 272?C ; FT-IR (KBr) ν 3414, 3338, 3009, 2961, 1621, 1511, 1243, 822 cm-1 ;1H NMR (400 MHz, CDCl3): δ 3.69 (3H, s, -OCH3), 4.14 (1H, bs, N-H), 6.14 (1H, s, methine-H), 6.75 (2H, d, J=8.4Hz, Ar-H), 6.82 (2H, d, J=8.5Hz, Ar-H), 6.98 (1H, t, J=7.5Hz, Ar-H), 7.13-7.18 (3H, m, Ar-H), 7.28-7.37 (4H, m, Ar-H), 7.75-7.85 (3H, m, Ar-H), 11.58 (1H, bs, OH) ppm; 13C NMR (100 MHz, CDCl3 ): δ 54.3, 61.4, 113.2, 113.6, 115.4, 118.9, 120.5, 120.8, 121.9, 125.8, 127.7, 128.1, 128.3, 128.6, 128.8, 130.5, 132.4, 145.8, 154.9, 158.8 ppm. Anal. Calcd. For C24H21NO2: C,81.12; H,5.97; N,3.96; Found: C,80.88; H,5.99; N,3.92%
1-[(3-Chloroyphenylamino)(phenyl)methyl]naphtholen-2-ol 5i
Colourless Crystals; Yield 85% ; mp: 114-116? C ; FT-IR (KBr) ν 3375, 3338, 3069, 2940, 1597, 1479, 1240, 758 cm-1 ;1H NMR (400 MHz, CDCl3): δ 4.18 (1H,bs,N-H), 6.18 (1H, s, methine), 6.64 (1H, dd, J=8.0Hz, Ar-H), 7.06 (1H, t, J=8.1Hz, Ar-H), 7.15 (1H, d, J=8.8Hz, Ar-H),7.31-7.35 (5H, m, Ar-H), 7.48 (2H, d, J=7.1Hz, Ar-H), 7.77-7.81 (3H, m, Ar-H), 10.89 (1H, bs, OH) ppm; 13C NMR (100 MHz, CDCl3 ): δ 61.8, 112.5, 113.6, 115.9, 118.9, 120.5, 120.8, 122.4, 125.9, 126.9, 127.5, 128.2, 128.3, 128.5, 129.2, 129.57, 129.2, 129.7, 130.7, 135.2, 139.6, 146.9, 154.9 ppm. Anal. Calcd. For C23H18ClNO: C,76.78; H,5.05; N,3.92; Found: C,76.54; H,5.15; N,3.88%
1-[(4-Methoxyphenylamino)(phenyl)methyl]naphtholen-2-ol 5j
Light brown solid; Yield 75% ; mp: 119-122?C ; FT-IR (KBr) ν 3398, 3328, 3027, 2931, 1623, 1511, 1238, 822 cm-1; 1H NMR (400 MHz, CDCl3): δ 3.71 (3H, s, -OCH3), 4.24 (1H, bs, N-H), 6.14 (1H, s, methine-H), 6.71-6.77 (4H, m, Ar-H), 7.18 (1H, d, J=8.8Hz, Ar-H), 7.29-7.40 (5H, m, Ar-H), 7.50 (2H, d, J=7.5Hz, Ar-H), 7.75-7.85 (3H, m, Ar-H), 11.98 (1H, bs, OH) ppm; 13C NMR (100 MHz, CDCl3 ): δ 54.6, 62.6, 112.8, 113.9, 116.9, 119.2, 120.5, 121.8, 125.8, 126.9, 127.9, 128.1, 128.3, 128.8, 130.6, 139.4, 140.8, 153.9, 155.8 ppm Anal. Calcd. For C24H21NO2: C,81.12; H,5.97; N,3.96; Found: C,80.92; H,5.93; N,3.90%
1-[(4-Nitrophenylamino)(phenyl)methyl]naphtholen-2-ol 5k
Yellow solid; Yield 88% ; mp: 154-156? C ; FT-IR (KBr) ν 3396, 3358, 3029, 2955, 1598, 1509, 1309, 1113, 768 cm-1 ; 1H NMR (400 MHz, CDCl3): δ 5.08 (1H, bs, N-H, disappeared in presence of D2O), 6.42 (1H, s, methine-H), 6.72 (2H, d, J=8.8Hz, Ar-H), 7.11 (1H, d, J=8.8Hz, Ar-H), 7.29-7.37 (4H, m, Ar-H), 7.42-7.45 (3H, m, Ar-H), 7.76-7.86 (3H, m, Ar-H), 8.01 (2H, d, J=8.8Hz, Ar-H), 11.67 (1H, bs, OH, disappeared in the presence of D2O) ppm; 13C NMR (100 MHz, CDCl3 ): δ 58.1, 113.1, 113.6, 118.4, 120.6, 122.5, 125.0, 126.3, 126.5, 127.5, 128.2, 128.5,128.6, 129.7, 130.7, 138.9, 139.7, 151.7, 153.2 ppm. Anal. Calcd. For C23H18N2O3: C,74.59; H,4.91; N,7.55; Found: C,74.34; H,4.95; N,7.48%
1-[(4-Fluorophenylamino)(phenyl)methyl]naphtholen-2-ol 5l
White solid ; Yield 85% ; mp: 111-112? C ; FT-IR (KBr) ν 3397, 3338, 3023, 2970, 1607, 1505, 1230, 816,753 cm-1 ;1H NMR (400 MHz, CDCl3): δ 4.15 (1H, bs, N-H), 6.12 (1H, s, methane-H), 6.69-6.73 (2H, m, Ar-H), 6.80-6-86 (2H, m, Ar-H), 7.46-7.47 (2H, m, Ar-H), 7.71-7.76 (3H, m, Ar-H), 11.69 (1H, bs, OH) ppm; 13C NMR (100 MHz, CDCl3 ): δ 61.8, 112.5, 114.6, 115.9, 118.9, 120.5, 120.8, 121.8, 125.9, 126.9, 127.2, 127.4, 127.9, 128.1, 128.4, 128.9, 129.2, 130.7, 137.4, 145.2, 155.6, 157.1,159.6 ppm. Anal. Calcd. For C23H18 FNO: C,80.46; H,5.28; N,4.09; Found: C,80.25; H,5.27; N,4.06%
1-[(3,4-Dichlorophenylamino)(phenyl)methyl]naphtholen-2-ol 5m
Brown solid; Yield 87% ; mp: 124-126? C ; FT-IR (KBr) ν 3403, 3353, 3062 ,2954, 1597, 1479, 1040, 748 cm-1 ;1 H NMR (400 MHz, CDCl3): δ 4.28 (1H, bs, N-H), 6.14 1H, s, methane-H), 6.54 (1H, dd, J=8.8Hz, J=2.4Hz, Ar-H), 6.81 (1H, d, J=2.4Hz, Ar-H), 7.09-7.14 (2H, m, Ar-H), 7.30-7.33 (4H, m, Ar-H), 7.35-7.44 (3H, m, Ar-H), 7.72-7.77 (3H, m, Ar-H), 10.55 (1H, bs, OH) ppm; 13C NMR (100 MHz, CDCl3 ): δ 60.8, 114.6, 116.9, 118.9, 120.8, 122.1, 122.4, 125.9, 126.8, 126.9, 127.7, 128.1, 128.2, 128.5, 129.2, 129.7, 130.7, 131.2, 139.2, 145.1, 154.9 ppm Anal. Calcd. For C23H17Cl2NO: C,70.07; H,4.35; N,3.55; Found: C,69.86; H,4.39; N,3.52%
1-[(4-Chlorophenyl)(3-chlorophenylamino)methyl]naphtholen-2-ol 5n
Brown solid; Yield 86%; mp: 123-124 ?C; FT-IR (KBr) ν 3398, 3346, 3063, 2965, 1598, 1485, 751cm-1 1H NMR (400MHz, CDCl3): δ 4.12 (1H, bs, N-H), 6.08 (1H, s, methine-H), 6.74 (2H, t, J=8.5Hz, Ar-H), 7.05-7.09 (2H, m, Ar-H), 7.22 (2H, d, J=8.4Hz, Ar-H), 7.32-7.36 (3H, m, Ar-H), 7.42 (2H, d, J=8.5Hz, Ar-H), 7.32-7.36 (3H, m, Ar-H), 7.42 (2H, d, J=8.5Hz, Ar-H), 7.89 (3H, d, J=8.9Hz, Ar-H), 11.52 (1H, bs, OH) ppm; 13C NMR (100 MHz, CDCl3): δ 63.4, 111.6, 114.3, 114.6, 118.0, 121.9, 122.9, 126.0, 126.9, 127.3, 123.4, 127.8, 128.5, 128.6, 128.9, 129.5, 132.6, 132.8, 134.4, 140.1, 151.8, 155.0, 157.4, 159.9 ppm. Anal. Calcd. For C23H17ClFNO: C,73.12; H,4.55; N,3.72; Found: C,72.93; H,4.57; N,3.71%
1-[(4-Methoxyphenylamino)(p-tolyl)methyl] naphthalen-2-ol 15o
Brown solid ; Yield 85%; mp: 115-117°C; FT-IR (KBr) ν 3401, 3339, 3013, 2959, 1605, 1509, 1252, 754cm-1; 1H NMR ( 400 MHz, CDCL3): δ 2.40 (3H, s, -CH3), 3.83 (3H, s,-OCH3), 4.11 (1H, bs ,N-H), 6.08 (1H, s, methine-H), 6.69-6.76 (2H, m, Ar-H), 6.93 (2H, d, J=8.9Hz, Ar-H), 7.15 (2H, d, J=8Hz, Ar-H), 7.25-7.28 (3H, m, Ar-H), 7.36 (2H, d, J=8.0Hz, Ar-H),7.75-7.79 (3H, m, Ar-H), 11.67 (1H, bs, OH) ppm; 13C NMR (100MHz, CDCl3 ): δ 19.7, 64.4, 83.5, 112.4, 115.4, 118.2, 122.2, 122.5, 124.4, 125.7,126.9, 127.4, 128.2, 128.3, 128.4, 131.4, 132.1, 136.4, 141.9, 143.5, 151.9 ppm. Anal. Calcd. For C25H23NO2: C,81.28; H,6.27; N,3.78; Found: C,81.09; H,6.29; N,3.76%.
1-[(4-Chlorophenyl)(4-fluorophenylamino)methyl] naphthalen-2-ol 15p
Colourless crystals; Yield 88%; mp: 164-165°C; FT-IR (KBr) ν 3432, 3355, 3061, 2961, 1624, 1506, 1221, 816, 772 cm-1; 1H NMR (400 MHz, CDCl3): δ 4.12 (1H, bs, N-H), 6.08 (1H, s, methine-H), 6.74 (2H, t, J=8.4Hz, Ar-H),7.04-7.08 (2H, m, Ar-H), 7.21 (2H, d, J=8.4Hz, Ar-H), 7.32-7.36 (3H, m, Ar-H), 7.42 (2H, d, J=8.4Hz), 7.88 (3H, d, J= 8.8Hz, Ar-H), 11.52 (1H, bs, OH) ppm; 13C NMR (100MHz,CDCl3): δ 63.4, 111.6, 114.3, 114.5, 117.9, 121.8, 129.5, 132.5, 132.8, 134.4, 140.0, 151.7, 155.0, 157.4, 159.9 ppm. Anal. Calcd. For C23H17CLFNO: C,73.12; H,4.55; N,3.72; Found:C,72.94; H,4.58; N,3.71%.
Conclusion
In conclusion , we have developed a high yielding protocol for the synthesis of aminocatalyst. This method operates efficiently under solvent benzene and demonstrates excellent substrate versatility, providing a valuable approach for the synthesis of aminonaphthol derivatives in organic chemistry .A novel series of arylaminonaphthols was synthesized through a one-pot three component reaction involving an aldehyde , an aromatic amine,and 2- naphthol, facilitated by DABCO under solvent benzene. This efficient methodology allows for the straight forward assembly of the desired compounds, show casing the effectiveness of DABCO as an organocatalyst and hightlighting the potential for practical applications in organic synthesis.
References
[1] Knapp S(1995) Synthesis of complex nucleoside antibiotics.Chem Rev 95:1859 1876. doi:10.1021/cr00038a006. [2] Mannhold R,Kubinyi H(2006) Nucleic acid drugs.In: Dingermann T, Steinhilber D, Folkers G(eds) Molecular biology in medicinal chemistry. Wiley, Weinhein. doi: 10.1002/3527602666. [3] Wang YK, Izawa T, Kobayashi S,Ohno M (1982) Stereocontrolled synthesis of (+)- negamycin from an acylic homoallylamine by 1,3-asymmetric induction. J Am Chem Soc 104:6465-6466. doi:10.1021/ja00387a060. [4] Cardellicchio C, Capozzi MAM , Naso F (2010) The Betti bases: the awakening of a sleeping beauty.Tetrahedron 21:507-517.doi : 10. 1016/j.tetasy.2010.03.020. [5] Elmendorf HG , Walls CD , Wolf C(2012) Compositions and methods for the treatment of giardiasis . US Patent 20, 120, 283,267. [6] Gerlach M, Maul C(2007) Substituted 1 and 2- naphthol Mannich bases. US Patent 7, 202,242B2. [7] Gyemant N, Engi H ,Schelz Z, Szatmari I, Toth D, Fulop F , Molnar J, de Witte P (2010) In vitro and in vivo multidrug resistance reversal activity by a Betti- base derivative of tylosin.Br J Cancer 103:178-185.doi:10.1038/sj.bje.6605716. [8] Shen AY, Tsai CT, Chen CL(1999) Synthesis and cardiovascular evaluation of N-substituted 1-aminomethyl-2-naphthols.Eur J Med Chem 34:877-882.doi:10.1016/S0223-5234(99)00204-4. [9] Szatmari I,Fulop F(2013) Synthesis, transformationsand applications of aminonaphthol derivatives prepared via modified Mannich reactions. Tetrahedron 69:1255-1278.doi:10.1016/j.tet.2012.11.055. [10] Salama TA (2013) Silicon - induced general, mild, and efficient one pot, three- component synthesis of amidoalkyl naphthol libraries. Synlett24:713-718.doi:10.1055/s-0032-131839211. Safari J, Zarnegar Z (2013) A magnetic nanoparticle-supported sulfuric acid as a highly efficient and reusable catalyst for rapid Synthesis of amidoalkylnaphthols.J Mil Catal A 379:269-276.doi:10.1016/j.molcata.2013.08.028. [11] Rostamizadeh S,Abdollahi F, Shadjou N, Amani AM(2013) MCM- 41-SO3H: a novel reusable nanocatalyst for synthesis of amidoalkylnaphthols under solvent - free conditions. Monatsh Chem 144:1191-1196.doi 10.1007/s00706-013-0936-4. [12] Lei ZK, Xiao L, Lu XQ, Huang H,Liu CJ(2013) Graphite supported perchloric acid ( HClO4- C):an efficient and recyclable heterogeneous catalyst for the one - pot synthesis of amidoalkylnaphthols. Molecules 18:1653-1659.doi:10.3390/ molecules18021653. [13] Das VK, Borah M, Thakur AJ(2013) Piper-betle-shaped nano- S-catalyzed synthesis of 1-amidoalkyl-2-naphthols under solvent -free reaction condition: A greener \" Nanoparticle-Catalyzed Organic Synthesis Enhancement\" approach. J Org Chem 78:3361-3366.doi:10.1021/jo302682k. [14] Csutortoki R, Szatmari I,Fulop F(2013) Syntheses of amidocarbamido- and carbamatoalkylnaphthols. Curr Org Synth 10:564-583. [15] Mulla SA, Salama TA, Pathan MY, Inamdar SM, Chavan SS(2012) Solvent-free,highly efficient one-pot multi-component synthesis of 1-amido and 1-carbamato-alkyl naphthols/phenols catalyzed by ethylammonium nitrate as reusable ionic liquid under neat reaction condition at ambient temperature. Tetrahedron Lett 54:672-675.doi:10.1016/j.tetlet.2012.12.004. [16] Kotadia DA, Soni SS(2012) Silica gel supported - SO3H functionalised benzimidazolium based ionic liquid as a mild and effective catalyst for rapid Synthesis of 1-amidoalkyl-2-naphthols. J Mol Catal A 353:44-49.doi:10.1016/j.molcata.2011.11.003. [17] ZolfigolMA , Khazaei A, Moosavi - Zare AR, Zare A, Khakyzadeh V (2011) Rapid synthesis of 1-amidoalkyl-2-naphthols over sulfonic acid functionalized imidazolium salts. Appl Catal A 400: 70-81.doi:10.1016/j.apcata.2011.04.013. [18] Tamaddon F, Bistgani JM (2011) [MeC(OH)2]+ClO4-: a new efficient organocatalyst for the preparation of 1- amido- and 1-carbamato- alkyl naphthols. Synlett2011:2947-2950.doi:10.1055/s-0031-1289906. [19] Mistry SR , Joshi RS, Maheria KC(2011) Zeolite H- BEA catalysed multicomponent reaction: one-pot synthesis of amidoalkylnaphthols -biologically active drug - like molecules. J Chem Sci 123:427-432.doi:10.1007/s12039-011-0095-2. [20] Shaterian HR, Yarahmadi H, Ghashang M (2008) Silica supported perchloric acid (HClO4-SiO2): an efficient and recyclable heterogeneous catalyst for the one - pot synthesis of . amidoalkyl-2-naphthols Tetrahedron 64:1263-1269.doi:10.1016/j.tet.2007.11.070. [21] Shaterian HR, Yarahmadi H, Ghashang M (2008) An efficient, simple and expedition synthesis of 1-amidoalkyl-2-naphthols as \"drug like\" molecules for biological screening. Bioorg Med Chem Lett 18:788-793.doi: 10.1016/j.tet.2007.11.070. [22] Karmakar B, Banerji J (2011) A competent pot and atom- efficient synthesis of Betti bases over nanocrystalline MgO involving a modified Mannich type reaction.Tetrahedron Lett 52:4957-4960.doi:10.1016/j.tetlet.2011.07.075. [23] Periasamy M, Anwar S, Reddy MN(2009) Simple and convenient methods for synthesis, resolution and application of aminonaphthols. Indian J Chem B 48: 1261- 1273. [24] Ghandi M, Olyaei A, Raoufmoghaddam S (2008) One - pot , three-component uncatalysedquantitive Synthesis of new aminonaphthols. Indian J Chem B 48: 1261-1273. [25] Oyaei A, Raoufmoghaddam S, Sadeghpour M, EbadzadehB(2010) Convenient and efficient method for the synthesis of N-heteroaryl aminonaphthols under solvent-free conditions. Chin J Chem 28:825-832.doi:10.1002/ cjoc.201090153. [26] Hosseinian A, Shaterian HR (2012) NaHSO4. H2O catalyzed multicomponent synthesis of 1-(benzothiazolylamino)methyl-2-naphthols under solvent - free conditions. Phosphorus Sulfur Silicon 187:1056-1063.doi:10.1080/10426507.2012.664221. [27] Buckley BR, Neary SP(2010) Organocatalysis. Annu Rep Prog Chem Sect B Org Chem 106:120-135.doi:10.1039/B927086H. [28] Grondal C, Jeanty M, Enders D(2010) Organocatalytic cascade reactions as a new tool in total synthesis. Nar Chem 2:167-178.doi:10.1038/nchem.539. [29] Marcelli T, van Maarseveen JH, Hiemstra H(2006) Cupreines and cupreidines:an emerging class of bifunctional cinchona organocatalysts. Angew Chem Int Ed 45:7496-7504. doi: 10.1002/anie. 200602318. [30] Liu G, Zhang S, Li H, Zhang T, Wang W(2011) Organocatalytic enantioselective Friedel-Crafts reactions of 1-naphthols with aldimines.Org Lett 13:838-831. doi:10.1021/oll02987n. [31] Ramachary DB, Babul Reddy G (2007) A new organocatalyst for Friedel-Crafts alkylation of 2- naphthols with isatins: application of an organi- click strategy for the cascade synthesis of highly functionalized molecules. Tetrahedron Lett 48: 7618-7623. doi:10.1016/j.tetlet.2007.08.129. [32] Shahrisa A, Safa KD, Esmati S(2014) Synthesis, spectroscopic and DFT studies of novel fluorescent dyes: 3-aminoimidazo[1,2-a] pyridines processing 4-pyrone moieties.Spectrochim Acta A 117:614-621.doi:10.1016/j saa.2013.09.056. [33] Shahrisa A, Esmati S, Miri R, Firuzi I, Edraki N, Nejati M (2013) Cytotoxic activity assessment, QSAR and docking study of novel bis- carboxamide derivatives of 4- pyrones synthesized by Ugi four- component reaction. Eur J Med Chem 66:388-399.doi:10.1016/j.ejmech.2013.05.045. [34] Shahrisa A, Esmati S (2013) Three novel sequential reactions for the facile synthesis of library of bisheterocycles possessing the 3-aminoimidazo[1,2-a] pyridine core catalysed by bismuth (III) chloride. Synlett24:595-602.doi:10.1055/s-0032-1318221. [35] ShahrisaA,MiriR,Esmati S, Saraei M, MehdipourAR,Sharifi M(2012) Synthesis and calcium channel antagonist activity of novel 1,4-dihydropyridine derivatives possesing 4-pyrone moieties. Med Chem Res 21:284-292.doi:10.1007/s00044-010-9534-8. [36] ShahrisaA, Esmati S, Nazari MG(2012) Boric acid as a mild and efficient catalyst for one - pot synthesis of 1-amidoalkyl-2-naphthols under solvent -free conditions. J Chem Sci 124:927-931.doi:10.1007/s12039-012-0285-6. [37] Dindulkar SD, Puranik VG,Jeong YT(2012) Supported copper triflate as an efficient catalytic system for the synthesis of highly functionalized 2-naphthol Mannich bases under solvent free condition. Tetrahedron Lett 53:4376-4380.doi:10.1016/j.tetlet.2012.06/022. [38] Ganesan SS, Rajendran N, Sundarakumar SI, Ganesan A, PemiahB(2013) 2-naphthol in glycerol: a versatile pair for efficient and convenient synthesis of aminonaphthols, naphtho-1,3-oxazines,and benzoxanthene. Synthesis 45:1564-1568.doi:10.1055/s-0033-1338430. [39] Saidi MR, Azizi N, Naimi- Jamal MR(2001) Lithium perchlorate assisted one- pot three-component aminoalkylation of electron- rich aromatic compounds. Tetrahedron Lett 43:8111-8113.doi:10.1016/S0040-4039(01)01732-4. [40] Katritzky AR, Abdel - Fattah AA, Tymoshenko DO, Belyakov SA, Ghiviriga I, Steel PJ(1999) Amino ( hetero) arylmethylation of phenols with N- [ alpha- amino( hetero) arylmethyl ] benzotriazoles. J Org Chem 64:6071-6075.doi: 10.1021/jo9903609. [41] Kumar A, Gupta MK, Kumar M( 2010) Non - ionic surfactant catalyzed synthesis of Betti bases in water . Tetrahedron Lett 51:1582-1584. doi:10.1016/ j.tetlet.2010.01.056.
Copyright
Copyright © 2025 Sushma Rani, M. K. Singh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66779
Publish Date : 2025-01-31
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

