Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
Orchids of Hottest Hot Spot in India: A Rich Source of Endophytes
Authors: Darsha S, Jayashankar M
DOI Link: https://doi.org/10.22214/ijraset.2024.63826
Certificate: View Certificate
Abstract
Western Ghats is one of the major tropical evergreen-forested regions in India and is one of the hottest hot spots in the World. Increasing urbanization as well as deforestation and thereby resulting habitat loss leads us to give a hand on conservation of such challenging biodiversity in these region. Aerides crispa and Cleisostoma tenuifolium are two vulnerable orchid plants from Kodagu, which is the heart of Western Ghats and are used as source of endophyte for the study. Isolation and molecular characterization of endophytic bacteria from the plants were studied to its genotypic level by comparing the PCR isolated 16S rRNA sequences. Nucleotide sequences were compared with the NCBI database and accession numbers for each bacterial strain were obtained from the GenBank. About 12 different bacteria were molecularly identified from these two orchids, A. crispa and C. tenuifolium. Orchids and microbes from orchids are less considered areas among researchers. Furthermore, the wide range of endophytes from such vulnerable plants speaks a lot about the necessity of bio-conservation of endophytes, orchids and thereby, harboring habitat of Western Ghats for the upcoming generation. However, vigorous trials were necessary to identify diversified endophytes from vulnerable plant habitats and so as a small step towards the biodiversity conservation of one of the most important biogeographic zones of the World.
Introduction
I. INTRODUCTION
As per UNESCO World heritage site, it is one of the eight “Hottest hot-spots” of biological diversity in the world. The mountain range is also known as “Sahyadri” begins from the Songadh town of Gujarat, south of Tapti river and covers approximately 1,600km through the states of Maharashtra, Goa, Karnataka, Kerala and Tamilnadu, ending at Marunthuvazhmalai at Swamithope, near Kanyakumari, the southern tip of India.
Kodagu district in Karnataka is located in the central part of the Western Ghats, situated in south India comprises 50% forest and agro-forestry area. Trees of Western Ghats harbor a variety of epiphytic orchids [1]. The forests of Western Ghats are known to be a varietal storehouse of economically important plants. The tropical climate, heavy rainfall from southwest monsoon and favorable soil factors made the area ideal for the rich biodiversity. The central Western Ghats area of Karnataka covers places viz., Kodagu, Hassan, Chikmagalur, Shivamogga, and Uttara Kannada [2].
Orchids are one of the most beautiful creations on the earth, comprising a unique group of plants valued mainly for the ornamental purpose and also for traditional medicine. These are one among the most threatened of all flowering plants. The Orchidaceae is one of the largest plant families with more than 25,000 species globally. These plants are known for their beauty and medicinal property. Although the large population of orchids is confined to their natural habitats, their number is decreasing because of high demand, habitat destruction and indiscriminate collection [3][4][5]. Evidence of plant-associated microorganisms found in the fossilized tissues of stems and leaves has revealed that endophyte-plant associations may have evolved from the time higher plants first appeared on the earth [6]. Endophytes are microbes that live within the plant tissue without causing any noticeable symptoms of disease. Endophytes have been found in all parts of plants including xylem and phloem. The majority of the endophytes have been isolated from trees, but only a few herbaceous plants and shrubs have been examined for the presence of endophytes. Rhizobium and other beneficial microbial diversity of three legumes plants that would help as biofertilizers for the crop from Fabaceae family [7].
Previous studies revealed about the endophytic fungal diversity [8] and a combination of endophytic bacteria and fungi [9] from vulnerable epiphytic orchids from Western Ghats. This investigation documents the diversity of residing endophytic bacteria in two plants via, A. crispa and C. tenuifolium from the heart of Western Ghats.
II. MATERIALS AND METHODS
Aerides crispa and Cleisostoma tenuifolium were used as specimens. Somwarpet agro-forestry region (located at 10.42°N 74.73°E latitude) of Kodagu, Karnataka which is the central part of Western ghats was the study area for plant collection. The parts of the plant can be randomly cut off with a disinfected sickle and placed separately in sterile polythene bags to avoid moisture loss. The materials were transported to the laboratory within 24 hours and stored at 4oC until the isolation procedures were completed.
The collected plant parts were thoroughly washed in running tap water to remove dirt and debris. Fresh healthy leaves and roots were selected for endophyte isolation. Epiphytes were removed from the surface by disinfecting the specimen by 70%ethanol for 1 minute, 4% sodium hypochlorite solution for 3 minutes; 70% ethanol for 30s and two rinses in sterilized distilled water. After removing the excess water, the leaf and root were excised into the size of 0.5X0.5 cm with the help of a sterile blade. A total of 50 segments were screened from each and these segments were placed in petri plates nutrient agar with chloramphenicol (150 mg/L).These plates were incubated at 28oC for 24-48 hours and after getting visible bacterial colonies. Each different colony was cultured separately by a streak plate method for obtaining pure culture. These pure cultures were maintained for further experiments. [10] [11].
Genomic DNA of bacterial culture were isolated by phenol-chloroform method according to Mora [12]. 16S rDNA obtained were quantified and amplified using the primers:Forward BSF: 5’GAGTTTGATCCTGGCTCAGG 3’; Reverse BSR: 5’ TCATCT GTCCC ACCTTCGGC 3’. The process of PCR was done using the set up; 10XPCRbuffer: 2.5 µl, MgCl2:2µl, dNTP’s mix (1mMeach):5 µl, Primer (10µM): F-0.5µl, R: 0.5µl, Taq polymerase (3U/µl): 0.3 µl DNA template (50ng/µl): 4µl. The PCR programme employed was as follows: primary denaturation for 5 minutes at 94ºC; 35 cycles of denaturation at 94ºC for 30s; annealing for 30 s, and extension at 72ºC for 1 min; and a final extension for 10 minutes at 72ºC.
Obtained sequences were compared with the known bacterial sequences from the NCBI database and then submitted to GenBank for accession number.
III. RESULTS
The plant specimen, ie, Aerides crispa and Cleisostoma tenuifolium before collection were shown inFigure 1 and Figure 3 which is present on the tree trunk. Those specimens after collection and before processing were shown in Figure 2 and Figure 4. From the epiphytic orchid plant Aerides crispa and Cleisostoma tenuifolium, different plants were used for the isolation of endophytic bacteria. A number of endophytes were obtained. We have selected a few for molecular identification. Those selected from A. crispa were AR1, AR2, AR3, AR4 and AL2; and from C. tenuifolium were BL1, BL2, BL3, BL4, BL5, BR1 and BR2 (names for temporary convenience).


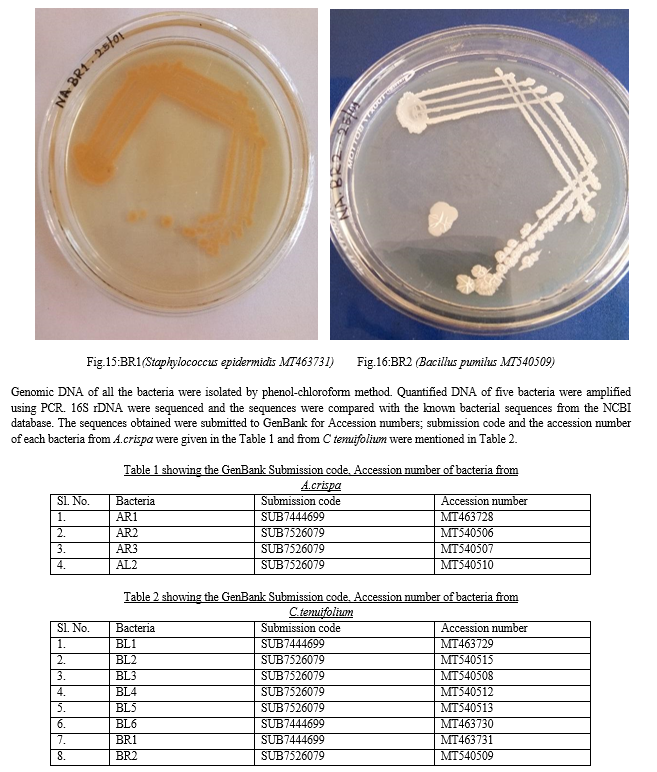
From the sequence analysis, twelve strains were studied: four bacterial strains from A. crispa were Bacillus pumilus MT463728, Bacillus megaterium MT540506, Lysinibacillus fusiformis MT540507, Bacillus cereus MT540510 (Fig.5 to Fig. 8).Eight bacterial endophytes from C tenuifolium were Bacillus subtilis MT463729, Psychrobacillus psychrodurans MT540515, Bacillus cereus MT540508, Bacillus safensis MT540512, Bacillus pumilus MT540513 Bacillus pumilus MT463730, Staphylococcus epidermidis MT463731, Bacillus pumilusMT540509 (Fig.9 to Fig16).
IV. DISCUSSION
Endophytic bacteria are found in roots, stems, leaves, seeds, fruits, tubers, ovules, and also inside legume nodules [7] . Pseudomonadaceae, Enterobac-teriaceae, Flavobacteriaceae, Burkholderiaceae, Xanthomonadaceae, and Bacillaceae families are well known plant-associated bacteria [13][14][15][16][17][18]. Joshi and Nongkhlaw [19] revealed a definite pattern in the diversity of culturable epiphytic bacteria, host-dependent colonization, microhabitat localization and biofilm formation which play a significant role in plant–microbe interaction. A novel endophytic filamentous bacterium strain was isolated from wild orchid Grosourdya appendiculata of Thailand [20]. The study of endophytic bacteria is important, not only for understanding their ecological role in their interaction with plants but also for their possible biotechnological applications, such as bioremediation. From this point of view, an interesting interaction between the endophytic bacterial community and glyphosate herbicide was observed during enrichment isolation. Only two bacterial species were recovered from the culture medium supplemented by glyphosate, Pseudomonas oryzihabitans and Burkholderia gladioli. The bacterium P. oryzihabitans was also recovered from total isolation and presented sensibility to glyphosate. This species has been isolated from different samples, such as soil, water, zones of rice cultivation [21], and moreover, soybean seeds [22] [23]. Suggested that these bacterial effects could be potentially useful to promote plant growth during seedling acclimatization in orchid species other than the species of origin [24].
Recently, host specificity has begun to be recognized, using Molecular Plant-Microbe Interactions molecular analysis based on the sequence of ribosomal genes [25] [26] [27]. Extensive information on the molecular mechanisms of other bacteria-plant interactions [28] [29] , there is only limited data on the endophyte-host molecular interactions. It would also be interesting to address whether some of the well-studied molecular mechanisms used by phytopathogenic bacteria [30] are to some extent shared with endophytes. Endophytic bacteria provide useful and rich models to study the genetic expression of bacteria in their natural niches or habitats (inside plants), which are more structured and variable than culture media under controlled laboratory conditions. Nevertheless, very little work has been done on this. Genomic projects are being performed on some endophytic bacteria, such as Azoarcus.
We have documented about twelve bacterial strains from two epiphytic orchids, A.crispa and C. tenuifolium. Compared to other methods, the molecular method of bacterial characterization was found better since it compared the genomes, thereby strain differentiation became possible. A vast number of bacterial strains from the small specimen speaks a lot about the importance of biodiversity. The number of studies related to endophytes is very low. Studies related to endophytes and orchids are sparse. Since, the present condition of decreasing forests, followed by reducing orchids leads us to study in this area. Because of the study, we are giving a small hand to biodiversity conservation and thereby conservation of the hottest hot spot, Western Ghats.
V. ACKNOWLEDGEMENT
We are grateful to Mangalore University for laboratory and technical assistance.
References
[1] Kumar P, Jalal JS, Rawat GS (2007), Orchidaceae, Chotanagpur, state of Jharkhand, India. Check List 3(4): 297-304 https://doi.org/10.15560/3.4.297 [2] Mahima VN., Nayak, MD, Subash C., Ramachandra TV (2016). Inventory of fishes of Gangavali estuary in Uttara Kannada, Karnataka state. Journal of the Marine Biological Association of India, 58(1), 69-74.https://doi.org/10.6024/jmbai.2016.58.1.1872-08 [3] Das A., Krishnaswamy J., Bawa, KS., Kiran, MC., Srinivas VN., Samba Kumar, NSK., Karanth, KU (2006). Prioritisation of conservation areas in the Western Ghats, India, Biological Conservation, 133(1), 16-31. https://doi.org/10.1016/j.biocon.2006.05.023 [4] Ayyanar, M., and Ignacimuthu S (2011). Ethnobotanical survey of medicinal plants commonly used by Kanitribals in Tirunelveli hills of Western Ghats, India. Journal of Ethnopharmacology, 134 (3), 851 – 864. https://doi.org/10.1016/j.jep.2011.01.029 [5] Marasini R and Joshi S (2013). Antibacterial and Antifungal Activity of Medicinal Orchids Growing in Nepal.Journal of Nepal Chemical Society, 29:104 -109. https://doi.org/10.3126/jncs.v29i0.9259 [6] Redecker D. (2000). Glomalean fungi from the Ordovician. Science, 289 (5486), 1920-1921. https://doi.org/10.1126/science.289.5486.1920 [7] Darsha S, Arivukarasu R, Jayashankar M, Saeed MA (2018). The Molecular identification of root nodule bacteria from edible crops of Fabaceae family, Kerala. Journal of Applied and Natural Science, 10(3), 1063-1065 https://doi.org/10.31018/jans.v10i3.1864 [8] Darsha S and Jayashankar M (2020a). Characterization of Endophytic Fungi from Western Ghat Orchid :Cleisostoma tenuifolium. International Journal of Scientific Research in Science and Technology, 7(6): 33-42 https://doi.org/10.32628/IJSRST20765 [9] Darsha S and Jayashankar M (2020b). Molecular characterization of bacterial and fungal endophytes associated with Vanda testacea, an orchid of Kodagu forest (Western Ghats), India. South Asian Journal of Experimental Biology; 10 (5): 292-300 https://doi.org.10.38150/sajeb.10(5).p292-300 [10] Kotain S., Vasudevan TG, Murali TS (2013) Fungal endophytes from two orchid species - pointer towards organ specificity. Czech Mycology 65(1), 89-101.https://doi.org/10.33585/cmy.65107 [11] Nalini MS., Sunayana, N and Prakash, HS (2014) Endophytic Fungal Diversity in Medicinal Plants of Western Ghats, India. International Journal of Biodiversity. 1-9. https://doi.org/10.1155/2014/494293 [12] Mora D., Fortina, MG., Parini C., Daffonchio D and Manachini PL. (2000). Genomic subpopulations within the species Pediococcus acidilactici detected by multilocus typing analysis: relationship between pediocin AcHPA-1 producing and non-producing strains. Microbiology, 146, 2027-2038. [13] Garbeva P., Overbeek L., Vuurde J., Elsas J (2001). Analysis of endophytic bacterial communities of potato by plating and denaturing gradient gel electrophoresis (DGGE) of 16S rDNA based PCR fragments. Microbial Ecology, 41, 369-383. [14] Halda-Alija L (2003). Identification of indole-3-acetic acid producing freshwater wetland rhizosphere bacteria associated with Juncus effuses L. Canadian Journal Microbiology, 49, 781–787. [15] Loiret F., Ortega E., Kleiner D., Ortega-Rodes P., Rodes R., Dong Z. (2004). A putative new endophytic nitrogen-fixing bacterium Pantoea sp. from sugarcane. Jourmal Applied Microbiology, 97, 504–511. [16] Park M., Kim C., Yang J., Lee H., Shin W., Kim S., Sa T (2005). Isolation and characterization of diazotrophic growth promoting bacteria from rhizosphere of agricultural crops of Korea. Microbiol. Res., 160, 127–133. [17] Unno Y., Okubo K., Wasaki J., Shinano T., Osaki M (2005). Plant growth promotion abilities and microscale bacterial dynamics in the rhizosphere of Lupin analysed by phytate utilization ability. Environmental Microbiology, 7, 396–404. https://doi.org/10.1080/0735-260291044223 [18] Young, C., Kampfer, P., Shen, F., Lai, W., Arun, A. (2005).Chryseobacterium formosense sp. nov., isolated from the rhizosphere of Lactuca sativa L. (garden lettuce). International Journal of Systemic and Evolutionary Microbiology, 55, 423–426. [19] Joshi SR and Nongkhlaw FMW (2014). Distribution pattern analysis of epiphytic bacteria on ethnomedicinal plant surfaces: A micrographical and molecular approach, Journal of Microscopy and Ultrastructure, 2(1), 34. https://doi.org/10.1016/j.jmau.2014.02.003 [20] Ngaemthao W, Pujchakarn T., Chunhametha S., Chanwit SC (2017) Verrucosispora endophytica sp. nov., isolated from the root of wild orchid (Grosourdya appendiculata (Blume) Rchb.f.) International Journal of Systematic and Evolutionary Microbiology, 67(12),5114- 5119. [21] https://doi.org/10.1099/ijsem.0.002425 Dussart L., Dupont JP., Zimmerlin I., Lacroix M., Saiter JM., Junter GA and Jouenne T (2003). Occurrence of sessile Pseudomonas oryzihabitans from a karstied chalk aquifer. Water Res. 2003, 37, 1593-1600. [22] Oehrle NW., Karr, DB., Kremer, RJ and Emerich D.W (2000). Enhanced attachment of Bradyrhizobium japonicum to soybean through reduced root colonization of internally seed borne microorganisms. Canadian Journal Microbiology, 46, 600-606. [23] Belimov, AA., Safronova, VI., Sergeyeva, TA., Egorova, TN., Matveyeva, VA., Tsyganov, VE., Borisov, AY., Tikhonovich, IA., Kluge C., Preisfeld A., Dietz, KJ and Stepanok, V V (2001). Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Canadian Journal Microbiology, 47, 642–652. [24] Faria DC., Dias ACF., Melo IS., Costa FE (2013). Endophytic bacteria isolated from orchid and their potential to promote plant growth. World Journal of Microbiology and Biotechnology, 29(2), 217-221. https://doi.org/10.1007/s11274-012-1173-4 [25] Jacquot E., Tuinen D, Gianinazzi S and Gianinazzi-Pearson V (2000). Monitoring species of arbuscular mycorrhizal fungi in plants and in soil by nested PCR: Application to the study of the impact of sewage sludge. Plant Soil, 226, 179-188. [26] Kjoller R and Rosendahl S (2000). Detection of arbuscular mycorrhizal fungi (Glomales) in roots by nested PCR and SSCP (single stranded conformation polymorphism). Plant Soil, 2000226, 189-196. [27] Rosenblueth M and Martínez-Romero E (2006). Bacterial Endophytes and Their Interactions with Hosts. The American Phytopathological Society, 19(8), 827–837. [28] Lugtenberg B (2015). Introduction to Plant-Microbe Interactions, Book: Principles of Plant-Microbe Interactions, 1-2. https://doi.org/10.1007/978-3-319-08575-3_1 [29] Oldroyd, GED., Downie, JA (2004). Calcium, kinases and nodulation signalling in legumes: Nature Reviews Molecular Cell Biology, 5 (7), 566-576.https://doi.org/10.1038/nrm1424 [30] Slot. K.A.E and Knogge, W A. (2002). Dual Role for Microbial Pathogen-Derived Effector Proteins in Plant Disease and Resistance. Critical Reviews in Plant Sciences, 21(3), 229- 271.
Copyright
Copyright © 2024 Darsha S, Jayashankar M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63826
Publish Date : 2024-07-31
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

