Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Optimizing MEP Design in Pharmaceutical Cleanroom with the Integration of 2D To 7D in BIM
Authors: Dr. Kirti Khandelwal, Dr. Namrata Lotia, Mr. Adil Sheikh, Mr. Prathamesh Charalwar, Mr. Md Shehzad Khan , Mr. Shabaz Ansari , Mr. Mohd. Shoyeb
DOI Link: https://doi.org/10.22214/ijraset.2024.62023
Certificate: View Certificate
Abstract
This paper examines the optimization of pharmaceutical cleanroom design using 2D to 7D Modelling in Building Information Modelling (BIM). The goal is to enhance the reliability, different methods, statistical data, and performance of pharmaceutical production plants by effectively allocating components within design constraints. Mathematical optimization techniques improve facility design, ensuring robustness and operational efficiency. The study proposes a reliable optimal design for air-conditioning systems in pharmaceutical cleanrooms, considering uncertainties in design parameters and operational strategies. Resilient cleanroom systems are developed, and adaptable to various conditions while maintaining high performance standards. Additionally, the research explores the application of experimental design methodologies in optimizing drug delivery systems, such as factorial and etc, to enhance the quality and efficiency of pharmaceutical products and processes. The integration of BIM in cleanroom design also allows for a sustainable and environmentally friendly approach, utilizing life cycle assessment methods to optimize facility design. This research underscores the potential of BIM in promoting sustainability in the pharmaceutical industry.
Introduction
I. INTRODUCTION
The integration of Building Information Modelling (BIM) technology in pharmaceutical cleanroom design has emerged as a transformative approach. This research focuses on optimizing Mechanical, Electrical, and Plumbing (MEP) systems in cleanrooms using advanced BIM capabilities. By exploring the synergies between MEP design and BIM, this study aims to enhance efficiency, sustainability, resolving factors and operational excellence in pharmaceutical manufacturing environments
MEP design and BIM technologies provide an opportunity to revolutionize the planning and execution of pharmaceutical cleanroom projects. This paper investigates the application of BIM tools to optimize MEP systems in cleanroom facilities. By utilizing digital modeling like 2D and 3D, simulation, and data integration, this research aims to streamline design processes, improve Productivity, and ensure in lack of time management.
The integration of BIM technology spanning from 2D to 7D has become a game-changer in cleanroom design for the pharmaceutical sector. This study focuses on optimizing MEP systems in pharmaceutical cleanrooms through the seamless integration of BIM technologies. By exploring the benefits of a multidimensional BIM approach, this research aims to provide insights into how advanced digital tools, different software and methods can enhance the design, construction, and maintenance of MEP systems in pharmaceutical manufacturing facilities.
II. METHODOLOGY
A. Understanding Pharmaceutical Cleanroom Requirements
Identify and understand the specific requirements of pharmaceutical cleanrooms, including cleanliness standards, temperature control, humidity levels, pressure differentials, air flow management, and lighting conditions.
Pharmaceutical cleanrooms are critical environments in the pharmaceutical industry that require strict adherence to specific requirements to maintain high standards of cleanliness and prevent contamination. Here are the key points from the provided sources regarding pharmaceutical cleanroom requirements.
Cleanroom Classification: Cleanrooms are classified based on the number of particles allowed per cubic meter, with ISO Class 8 cleanrooms having specific particle limits.
|
ISO 14644-1 |
FS 209E |
ACPH |
|
ISO 5 |
CLASS 100 (A) |
240-360 ACPH |
|
ISO 6 |
CLASS 1,000 (B) |
90-180 ACPH |
|
ISO 7 |
CLASS 10,000 (C) |
30-60 ACPH |
|
ISO 8 |
CLASS 100,000 (D) |
15-25 ACPH |
B. Factors Affecting
After review the previous research paper we identify the major factor which affect the cleanroom design and optimize the factors by using the specific methods.
- Air Quality and Contaminant Control
- Ventilation System Design
- Compliance with Standard
- Material Selection and Construction
- Workflow and Space Management
- Cost Management
- Pressurization and Protrusions
III. CASE STUDY
The cooling load temperature difference (CLTD) method is a widely used technique for predicting the cooling load of a building. In the context of MEP design for pharmaceutical applications, the CLTD method can be utilized to accurately size the HVAC system for the specific cooling requirements of pharmaceutical facilities.
- *Step 1: Calculate the CLTD*
The CLTD is calculated as the temperature difference between the outdoor design temperature and the desired indoor temperature. This value is crucial for sizing the HVAC system accurately for pharmaceutical facilities, where precise temperature control is often necessary.
2. *Step 2: Incorporate CLTD into HVAC System Design*
The calculated CLTD value is then incorporated into the HVAC system design process to ensure that the system is sized to meet the specific cooling requirements of the pharmaceutical facility. This includes considering factors such as the type and quantity of equipment, the number of occupants, and the desired indoor temperature.
3. *Step 3: Consider Pharmaceutical-Specific Requirements*
Pharmaceutical facilities often have unique requirements, such as cleanroom conditions, temperature control for sensitive materials, and air quality standards. The MEP design process must take these factors into account when designing the HVAC system to ensure that it meets the specific needs of the facility.
4. *Step 4: Optimize System Design*
The CLTD value and pharmaceutical-specific requirements are used to optimize the HVAC system design. This may involve selecting specific types of chillers, air handling units, and ductwork, as well as designing the system to minimize energy consumption while maintaining the required indoor conditions.
5. *Step 5: Validate and Refine the Design*
The final step is to validate and refine the HVAC system design through simulation and analysis. This ensures that the system is capable of maintaining the required indoor conditions while minimizing energy consumption and operating costs.
By following these steps, the CLTD method can be effectively used in MEP design for pharmaceutical applications to ensure that the HVAC system is accurately sized and optimized for the specific cooling requirements of the facility.
There are two distinct components of the air conditioning load;
- The sensible load (heat gain)
- The latent load (water vapor gain)
A. Sensible Load
Sensible heat gain is the direct addition of heat to a space, which shall result in increase in space temperatures. The factors influencing sensible cooling load:
- Solar heat gain through building envelope (exterior walls, glazing, skylights, roof, floors over crawl space)
- Partitions (that separate spaces of different temperatures)
- Ventilation air and air infiltration through cracks in the building, doors, and windows
- People in the building
- Equipment and appliances operated in the summer
- Lights
B. Latent Load
A latent heat gain is the heat contained in water vapor. Latent heat does not cause a temperature rise, but it constitutes a load on the cooling equipment. Latent load is the heat that must be removed to condense the moisture out of the air. The sources of latent heat gain are:
- People (breathing)
- Cooking equipment
- Housekeeping, floor washing etc.
- Appliances or machinery that evaporates water
- Ventilation air and air infiltration through cracks in the building, doors, and windows. The total cooling load is the summation of sensible and latent loads.
C. Statistical Data
By using the CLTD method the maximum load for the
July and August month was calculated to be 9.87 Tons at 5pm in the evening. The breakdown of individual external loads as well as monthly load calculated are shown below.
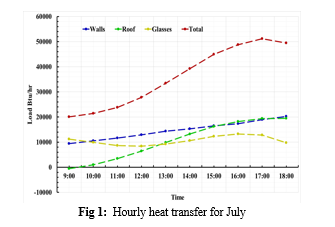
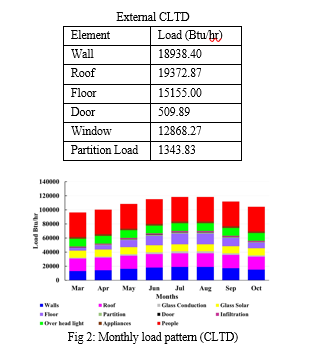
IV. COMPUTATIONAL FLUID DYNAMICS (CFD)
In HVAC system, CFD analysis can help predict airflow and temperature distribution, and assess acoustic properties. It can also help engineers optimize design to improve air distribution and thermal comfort.
A. CFD Result
The simulations were performed for various A/C Ton configurations for March to October. For assisting the simulation as well as the analysis, it was assumed that the human comfort conditions to begin below 23.5ºC and final desired inner targeted temperature conditions to be multiple one; being 22.5ºC, 22ºC and 21.5ºC.
Considering the response time, in all cases of desired inner temperature, it was found that the performance ranged from the highest A/C configuration i.e. 11 Tons to cool the quickest while the 8 Tons to be the slowest, which was obvious. The 8 Ton A/C configuration was not able to cool down the temperature of the space to 21.5ºC. The 8 Ton A/C configuration suffered significant performance loss in the month of July and August being able to drop the temperature to human comfort range only after the 30-40 minute mark, yet never able to attain the 21.5ºC. However, in other months overall it is able to drop temperature to human comfort range within 15-25 minutes.
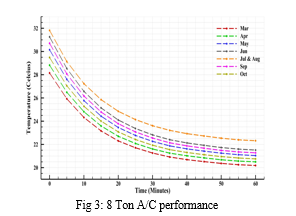
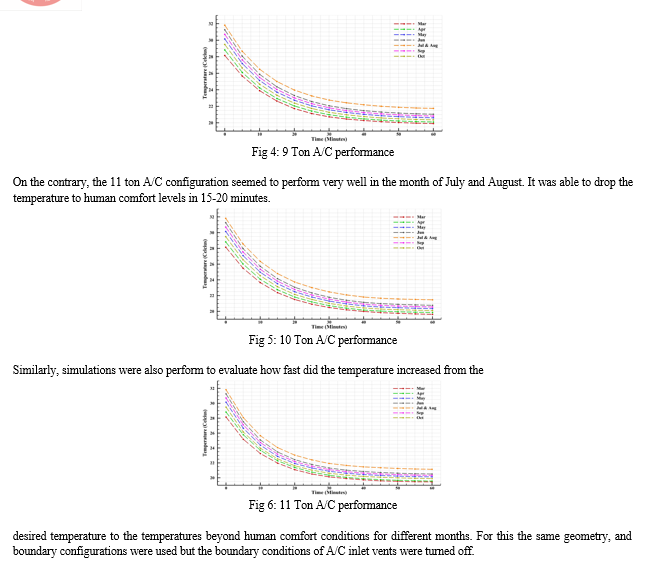
V. RECOMMENDATIONS AND FUTURE SCOPE
Building Information Modeling (BIM) is a digital representation of the physical and functional characteristics of a building. It integrates various systems including MEP design to optimize building performance and efficiency.
Pharmaceutical cleanrooms require stringent environmental controls to ensure the quality and safety of pharmaceutical products. MEP design plays a crucial role in achieving the required cleanliness levels and maintaining optimal conditions within cleanrooms.
Recommendations for pharmaceutical cleanrooms include the use of advanced HVAC systems for precise temperature and humidity control, air filtration systems to remove contaminants, and efficient lighting systems to minimize shadows and improve visibility.
Future studies should focus on the integration of renewable energy sources to reduce energy consumption, the use of smart sensors for real-time monitoring and control, and the implementation of advanced automation systems for increased operational efficiency.
Conclusion, the implementation of MEP design through BIM offers significant benefits for pharmaceutical cleanrooms, including improved environmental control, energy efficiency, and overall performance. By following the recommendations outlined in this report and continuing to explore future directions, pharmaceutical cleanrooms can enhance their operations and ensure the highest standards of product quality and safety.
VI. RESULT AND DISCUSSION
A. Results
The pharmaceutical cleanroom designed using MEP systems through BIM showed significant improvements in controlling temperature, humidity, and air quality. The integrated MEP systems helped in achieving cleanroom classifications as per industry standards, ensuring the production of high-quality pharmaceutical products. The BIM model facilitated the identification of potential clashes and design conflicts, leading to timely resolutions and cost savings during the construction phase.
B. Discussion
The use of BIM in conjunction with MEP design for pharmaceutical cleanrooms offers several advantages, including improved efficiency, accuracy, and collaboration among project teams. The visual representation of cleanroom designs in 3D models allows for better decision-making and problem-solving throughout the project lifecycle. Additionally, the integration of MEP systems early in the design process helps in optimizing cleanroom performance and reducing operational costs in the long run.
Conclusion
The integration of MEP design with BIM technology has significantly improved the efficiency and effectiveness of pharmaceutical cleanroom designs. By utilizing BIM software, engineers and designers can create detailed 3D models of cleanroom facilities, allowing for better visualization and coordination of various MEP systems. This has led to a more streamlined design process, resulting in reduced errors, improved communication, and enhanced overall performance of the cleanroom. One of the main conclusions of this report is that the use of BIM technology in conjunction with MEP design has led to cost savings and accelerated project timelines in pharmaceutical cleanroom construction. By identifying potential clashes and design inconsistencies early on, project delays and costly changes during construction can be minimized. This has helped pharmaceutical companies to bring their products to market faster and more efficiently. Additionally, the integration of MEP design with BIM technology has resulted in improved energy efficiency and sustainability of pharmaceutical cleanrooms. By optimizing HVAC systems, lighting, and other MEP components, cleanroom facilities can operate at peak performance while reducing energy consumption and environmental impact. This not only benefits the bottom line of pharmaceutical companies but also contributes to their corporate social responsibility efforts. In conclusion, the optimization of pharmaceutical cleanrooms through MEP design with the use of BIM technology has proven to be a game-changer in the industry. The benefits of this approach include cost savings, accelerated project timelines, improved energy efficiency, and overall enhanced performance of cleanroom facilities. Moving forward, further research and development in this area are warranted to continue advancing the design and construction of pharmaceutical cleanrooms for the benefit of patients and the pharmaceutical industry as a whole.
References
[1] Cai, L. Q. (2020). Research on the Application of BIM Technology in HVAC Design. Residential and Real Estate. [2] Lu, L., & Zong,T. (2019). Application analysis of BIM technology in HVAC design Zhu, L. Q. (2023). [3] Application of BIM technology in building water supply and drainage and HVAC design. Scientific and technological innovation and application. [4] S. Azhar, M. Khalfan, T. Maqsood, Building information modelling (BIM): now and [5] beyond, Construction Economics and Building [6] Dallasega, P., Schulze, F., Revolti, A., & Martinelli, M. (2021). Augmented Reality to increase efficiency of MEP construction: A case study. ISARC Proceedings [7] Gu, N., & London, K. (2010). Understanding and facilitating BIM adoption in the AEC industry. Automation in Construction [8] Eldeep, A. M., Farag, M. A. M., & Abd El-hafez, L. M. (2022). Using BIM as a lean management tool in construction processes – A case study. Ain Shams [9] Engineering Journal [10] Wang, W. L., Zhou, G. M., Yao, H. Z., & Yuan, C. S. (2021). Analysis on the application of BIM technology in HVAC system design.
Copyright
Copyright © 2024 Dr. Kirti Khandelwal, Dr. Namrata Lotia, Mr. Adil Sheikh, Mr. Prathamesh Charalwar, Mr. Md Shehzad Khan , Mr. Shabaz Ansari , Mr. Mohd. Shoyeb. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET62023
Publish Date : 2024-05-13
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

