Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Introduction
- Conclusion
- References
- Copyright
Pharmacological Assessment of Withania Somnifera
Authors: Krithi Sastry, Dr. Estuti Chandra, Ms. Poornima Srinivas, Dr. Pratima Abhay Kochhar
DOI Link: https://doi.org/10.22214/ijraset.2025.66870
Certificate: View Certificate
Abstract
Introduction
I. INTRODUCTION
Withania somnifera, commonly known as Ashwagandha, is a prominent herb in traditional Ayurvedic medicine, renowned for its adaptogenic, anti-inflammatory, and antioxidant properties. It is often used to combat stress, anxiety, and fatigue, as well as to improve physical endurance and cognitive function. The active compounds in Withania somnifera, including withanolides, alkaloids, and steroidal lactones, contribute to its pharmacological activities, influencing various physiological systems.
There are evidence supporting its potential therapeutic benefits in managing conditions such as depression, neurodegenerative diseases, and metabolic disorders. Additionally, Withania somnifera has been explored for its immunomodulatory effects, promoting the body's ability to resist infections and inflammation.
II. FEATURES OF ASHWAGANDHA
- Molecular Formula- C28H38O6
- Synonyms- Withanolide D, 30655-48-2, WITHANIA SOMNIFERA, XY366XV8JT, Dihydrowithanolide D
- Molecular Weight- 470.6 g/mol
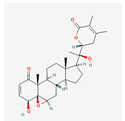
Fig: 2-D structure of Withania Somnifera
III. PROPERTIES OF ASHWAGANDHA
- Anti-inflammatory Properties: The effectiveness of ashwagandha in a variety of rheumatologic conditions may be due in part to its anti-inflammatory properties, which have been studied by several authors. In a study by Anbalagan et al, powdered root of WS (1 g/kg suspended in 2% gum acacia, 50 mg/mL) was given orally one hour before the induction of inflammation by injection of Freund’s complete adjuvant in rats and continued daily for three days; et al found WS caused dose-dependent suppression of α2-macroglobulin (an indicator for anti-inflammatory drugs) in the serum of rats inflamed by sub-plantar injection of carrageenan suspension. The doses of WS root powder were 500, 1000, 1500, or 1200 mg/kg given as suspension orally 3-4 hours prior to induction of inflammation. Maximum effect (about 75%) was seen at 1000 mg/kg. Actual measurements of inflammation were not conducted
- Anti-Cancer Properties: It is used in Ayurvedic medicine and has garnered significant attention for its potential anticancer properties. Withanolides have been shown to induce programmed cell death (apoptosis) in cancer cells. This process involves the activation of pro-apoptotic pathways, such as the mitochondrial pathway, and the inhibition of anti-apoptotic proteins, which leads to the elimination of malignant cells. Ashwagandha has also been reported to suppress the formation of new blood vessels (angiogenesis) that supply nutrients to tumours. By inhibiting angiogenesis, Ashwagandha can limit tumour growth and metastasis.
- Anti-Stress Effect: One of the primary mechanisms through which Ashwagandha exerts its anti-stress effects is by lowering cortisol, the "stress hormone." Cortisol is produced by the adrenal glands in response to stress, and chronically high levels of cortisol are associated with anxiety, depression, and various other stress-related conditions. Ashwagandha has been shown to reduce cortisol levels, which helps mitigate the physiological effects of stress, such as increased heart rate, high blood pressure, and immune suppression.
- Immunomodulatory Properties: The use of WS as a general tonic to increase energy and prevent disease may be partially related to its effect on the immune system. Glycowithanolides and a mixture of sitoindosides IX and X isolated from WS were evaluated for their immunomodulatory and central nervous system effects (antistress, memory, and learning) in Swiss mice (15-25 g, 5-6 months old) and Wistar strain albino rats (120-150 g and 250-300 g).31 Both materials produced statistically significant mobilization and activation of peritoneal macrophages, phagocytosis, and increased activity of the lysosomal enzymes. Both compounds (50-200 mg/kg orally) also produced significant antistress activity in albino mice and rats, and augmented learning acquisition and memory retention in both young and old rats.
IV. METHODOLOGY
Phytochemical Prediction-: IMPPAT (Indian Medicinal Plants, Phytochemistry, and Therapeutics) is a comprehensive, curated database that provides detailed information on over 4,000 Indian medicinal plants, their phytochemical compositions, and associated therapeutic uses. This resource aims to facilitate natural product-based drug discovery by offering insights into the chemical properties and medicinal applications of these plants
Swiss ADME-: SwissADME is an online tool that provides a range of computational methods to predict the physicochemical properties, pharmacokinetics, and drug-likeness of small molecules. It is particularly useful in the early stages of drug discovery to assess whether a compound has the potential to become a drug or therapeutic agent.
A. Components of Swiss ADME
- Prediction of Physicochemical Properties: Molecular weight, solubility, and logP (lipophilicity), Hydrogen bond donors and acceptors
- Pharmacokinetic Predictions:
- Absorption: How likely a molecule is to be absorbed in the gastrointestinal tract (e.g., through oral administration).
- Distribution: How the molecule spreads throughout the body, including the blood-brain barrier (BBB) penetration prediction.
- Metabolism: Likely sites for enzymatic metabolism, typically involving cytochrome P450 enzymes.
- Excretion: The way the molecule is cleared from the body (e.g., renal excretion).
- Drug-likeness Assessment: SwissADME evaluates a compound's "drug-likeness" based on Lipinski's Rule of Five, Veber's Rules, and other criteria, which help predict whether a molecule has properties like known drugs.
Table 1- Phytochemical Data of Withania Somnifera
|
Sr. no |
Plant part |
Phytochemical identifier |
Phytochemical name |
Smiles |
|
1 |
leaf |
OCC1=C(C)[C@@H]([C@@H](OC1=O)[C@H]([C@@]1(O)CC[C@@H]2[C@]1(C)CC[C@H]1[C@H]2CC=C2[C@]1(C)C(=O)C=CC2)C)O |
||
|
2 |
leaf |
O=C1O[C@H]([C@H](C(=C1C)C)O)[C@H]([C@H]1CC[C@@H]2[C@]1(C)CC[C@H]1[C@H]2[C@@H]2O[C@@H]2[C@@]2([C@]1(C)C(=O)C=CC2)O)C |
||
|
3 |
leaf |
CC1=C(C)C(=O)O[C@H](C1)[C@@]([C@@]1(O)C[C@H]2[C@@]3([C@]1(C)CC[C@H]1[C@H]3CC=C3[C@]1(C)C(=O)C=CC3)O2)(O)C |
||
|
4 |
leaf |
CC(=O)C[C@H]1CCCN1C |
||
|
5 |
leaf |
CC1=C(C)C(=O)O[C@H](C1)[C@@]([C@H]1CC[C@@H]2[C@]1(C)CC[C@H]1[C@H]2C[C@@H]2[C@]3([C@]1(C)C(=O)C=C[C@@H]3O)O2)(O)C |
||
|
6 |
leaf |
OCC1=C(C)C[C@@H](OC1=O)[C@H]([C@H]1CC[C@@H]2[C@]1(C)CC[C@H]1[C@H]2C[C@@H]2[C@]3([C@]1(C)C(=O)C=C[C@@H]3O)O2)C |
||
|
7 |
leaf |
CC1=C(C)C(=O)O[C@H](C1)[C@@]([C@]1(O)CC[C@@]2([C@]1(C)CC[C@H]1[C@H]2C[C@@H]2[C@]3([C@]1(C)C(=O)C=CC3)O2)O)(O)C |
||
|
8 |
leaf |
c1ccc(cc1)c1cnn2c1CCC2 |
||
|
9 |
leaf |
CC1=C(C)C(=O)O[C@H](C1)[C@@]([C@H]1CC[C@@]2([C@]1(C)CC[C@H]1[C@H]2CC=C2[C@]1(C)C(=O)C=CC2)O)(O)C |
||
|
10 |
leaf |
CC1=C(C)C(=O)O[C@H](C1)[C@H]([C@@]1(O)CCC2=C3[C@H](CC[C@]12C)[C@@]1(C)C(=O)C=C[C@@H](C1=CC3)O)C |
Table 2- Physiochemical Properties of Phytochemicals of Withania Somnifera
|
Phytochemical name |
Formula |
MW |
#Heavy atoms |
#Aromatic heavy atoms |
Fraction Csp3 |
#Rotatable bonds |
#H-bond acceptors |
#H-bond donors |
MR |
TPSA |
|
C28H38O6 |
470.6 |
34 |
0 |
0.71 |
3 |
6 |
3 |
129.21 |
104.06 |
|
|
C28H38O6 |
470.6 |
34 |
0 |
0.79 |
2 |
6 |
2 |
127.49 |
96.36 |
|
|
C28H36O6 |
468.58 |
34 |
0 |
0.71 |
2 |
6 |
2 |
127.09 |
96.36 |
|
|
C8H15NO |
141.21 |
10 |
0 |
0.88 |
2 |
2 |
0 |
45.47 |
20.31 |
|
|
C28H38O6 |
470.6 |
34 |
0 |
0.79 |
2 |
6 |
2 |
127.53 |
96.36 |
|
|
C28H38O6 |
470.6 |
34 |
0 |
0.79 |
3 |
6 |
2 |
127.49 |
96.36 |
|
|
C28H38O7 |
486.6 |
35 |
0 |
0.79 |
2 |
7 |
3 |
128.77 |
116.59 |
|
|
C12H12N2 |
184.24 |
14 |
11 |
0.25 |
1 |
1 |
0 |
56.58 |
17.82 |
|
|
C28H38O5 |
454.6 |
33 |
0 |
0.71 |
2 |
5 |
2 |
128.08 |
83.83 |
|
|
C28H36O5 |
452.58 |
33 |
0 |
0.64 |
2 |
5 |
2 |
127.57 |
83.83 |
Table 3- Lipophilicity of Phytocompounds of Withania Somnifera
|
Phytochemical name |
iLOGP |
XLOGP3 |
WLOGP |
MLOGP |
Silicos-IT Log P |
Consensus Log P |
|
3.36 |
2.88 |
3.26 |
2.67 |
3.59 |
3.15 |
|
|
3.69 |
3.55 |
3.35 |
2.75 |
3.65 |
3.4 |
|
|
3.72 |
2.43 |
3.56 |
2.67 |
4.16 |
3.31 |
|
|
2.11 |
0.47 |
0.68 |
0.76 |
1.29 |
1.06 |
|
|
3.8 |
3.12 |
3.5 |
2.75 |
3.78 |
3.39 |
|
|
3.24 |
3.83 |
3.35 |
2.75 |
3.93 |
3.42 |
|
|
3.21 |
1.74 |
2.75 |
1.95 |
3.68 |
2.67 |
|
|
2.1 |
2.22 |
2.5 |
2.34 |
2.68 |
2.37 |
|
|
3.74 |
3.47 |
4.43 |
3.48 |
4.33 |
3.89 |
|
|
3.34 |
2.64 |
4.35 |
3.4 |
4.58 |
3.66 |
Table 4- Solubility of Phytocompounds of Withania Somnifera
|
Phytochemical name |
ESOL Log S |
ESOL Solubility (mg/ml) |
ESOL Solubility (mol/l) |
ESOL Class |
Ali Log S |
Ali Solubility (mg/ml) |
Ali Solubility (mol/l) |
Ali Class |
Silicos-IT LogSw |
Silicos-IT Solubility (mg/ml) |
Silicos-IT Solubility (mol/l) |
Silicos-IT class |
|
-4.37 |
1.99E-02 |
4.23E-05 |
Moderately soluble |
-4.73 |
8.86E-03 |
1.88E-05 |
Moderately soluble |
-3.7 |
9.41E-02 |
2.00E-04 |
Soluble |
|
|
-4.86 |
6.46E-03 |
1.37E-05 |
Moderately soluble |
-5.26 |
2.59E-03 |
5.51E-06 |
Moderately soluble |
-3.55 |
1.34E-01 |
2.84E-04 |
Soluble |
|
|
-4.14 |
3.36E-02 |
7.18E-05 |
Moderately soluble |
-4.1 |
3.75E-02 |
8.01E-05 |
Moderately soluble |
-4.21 |
2.88E-02 |
6.15E-05 |
Moderately soluble |
|
|
-0.88 |
1.86E+01 |
1.32E-01 |
Very soluble |
-0.47 |
4.84E+01 |
3.42E-01 |
Very soluble |
-1.2 |
8.97E+00 |
6.35E-02 |
Soluble |
|
|
-4.59 |
1.21E-02 |
2.56E-05 |
Moderately soluble |
-4.81 |
7.25E-03 |
1.54E-05 |
Moderately soluble |
-3.78 |
7.85E-02 |
1.67E-04 |
Soluble |
|
|
-4.97 |
5.01E-03 |
1.07E-05 |
Moderately soluble |
-5.55 |
1.33E-03 |
2.82E-06 |
Moderately soluble |
-3.79 |
7.54E-02 |
1.60E-04 |
Soluble |
|
|
-3.82 |
7.35E-02 |
1.51E-04 |
Soluble |
-3.81 |
7.62E-02 |
1.57E-04 |
Soluble |
-3.85 |
6.79E-02 |
1.40E-04 |
Soluble |
|
|
-2.9 |
2.34E-01 |
1.27E-03 |
Soluble |
-2.23 |
1.09E+00 |
5.90E-03 |
Soluble |
-3.76 |
3.22E-02 |
1.75E-04 |
Soluble |
|
|
-4.71 |
8.81E-03 |
1.94E-05 |
Moderately soluble |
-4.91 |
5.56E-03 |
1.22E-05 |
Moderately soluble |
-4.5 |
1.42E-02 |
3.13E-05 |
Moderately soluble |
|
|
-4.18 |
3.01E-02 |
6.65E-05 |
Moderately soluble |
-4.05 |
4.02E-02 |
8.89E-05 |
Moderately soluble |
-4.71 |
8.91E-03 |
1.97E-05 |
Moderately soluble |
Table 5- Pharmacokinetics of Phytocompounds of Withania Somnifera
|
Phytochemical name |
GI absorption |
BBB permeant |
Pgp substrate |
CYP1A2 inhibitor |
CYP2C19 inhibitor |
CYP2C9 inhibitor |
CYP2D6 inhibitor |
CYP3A4 inhibitor |
log Kp (cm/s) |
|
High |
No |
Yes |
No |
No |
No |
No |
No |
-7.13 |
|
|
High |
No |
Yes |
No |
No |
No |
No |
No |
-6.65 |
|
|
High |
No |
Yes |
No |
No |
No |
No |
No |
-7.43 |
|
|
High |
No |
No |
No |
No |
No |
No |
No |
-6.83 |
|
|
High |
No |
Yes |
No |
No |
No |
No |
No |
-6.96 |
|
|
High |
No |
Yes |
No |
No |
No |
No |
No |
-6.45 |
|
|
High |
No |
Yes |
No |
No |
No |
No |
No |
-8.03 |
|
|
High |
Yes |
Yes |
Yes |
No |
No |
No |
No |
-5.85 |
|
|
High |
No |
Yes |
No |
No |
No |
No |
No |
-6.61 |
|
|
High |
No |
Yes |
No |
No |
No |
No |
No |
-7.19 |
Table 6- Drug likeliness properties of Phytocompounds of Withania Somnifera
|
Phytochemical name |
Lipinski #violations |
Ghose #violations |
Veber #violations |
Egan #violations |
Muegge #violations |
Bioavailability Score |
|
0 |
1 |
0 |
0 |
0 |
0.55 |
|
|
0 |
1 |
0 |
0 |
0 |
0.55 |
|
|
0 |
0 |
0 |
0 |
0 |
0.55 |
|
|
0 |
1 |
0 |
0 |
1 |
0.55 |
|
|
0 |
1 |
0 |
0 |
0 |
0.55 |
|
|
0 |
1 |
0 |
0 |
0 |
0.55 |
|
|
0 |
2 |
0 |
0 |
0 |
0.55 |
|
|
0 |
0 |
0 |
0 |
1 |
0.55 |
|
|
0 |
1 |
0 |
0 |
0 |
0.55 |
|
|
0 |
0 |
0 |
0 |
0 |
0.55 |
Table 7- Medicinal chemistry properties of Phytocompounds of Withania Somnifera
|
Phytochemical name |
PAINS #alerts |
Brenk #alerts |
Leadlikeness #violations |
Synthetic Accessibility |
|
0 |
1 |
1 |
6.43 |
|
|
0 |
1 |
2 |
6.51 |
|
|
0 |
2 |
1 |
6.78 |
|
|
0 |
0 |
1 |
1.8 |
|
|
0 |
1 |
1 |
6.85 |
|
|
0 |
1 |
2 |
6.83 |
|
|
0 |
1 |
1 |
6.9 |
|
|
0 |
0 |
1 |
2.2 |
|
|
0 |
1 |
1 |
6.28 |
|
|
0 |
1 |
1 |
6.15 |
Table 1 describes the phytochemical data of a certain selected phytocompounds in the leaf part of the plant Withnia Somnifera that contains a unique Phytochemical identifier ID dedicated to the compounds from the IMPPAT database along with the phytochemical name and the SMILES (The Simplified Molecular Input Line Entry System (SMILES) is a specification in the form of a line notation for describing the structure of chemical species using short ASCII strings.)
Table 2 describes the Physiochemical properties of the phytocompounds that includes criteria such as the molecular formula and the molecular weight along with normal and aromatic heavy atoms, rotatable molecules, and the hydrogen bond acceptors.
Table 3 enlists the lipophilicity of the phytocompounds. Lipophilicity refers to the ability of a compound to dissolve in fats, oils, and other non-polar solvents, rather than in water (which is polar). It is an important property that indicates how well a substance interacts with lipids (fatty substances) in the body, such as cell membranes. Lipophilicity is often measured by the partition coefficient (logP), which compares the solubility of a compound in a lipophilic solvent (like octanol) to that in water. Swiss ADME has five openly accessible models to determine the lipophilicity of compounds: XLOGP3, WLOGP, MLOGP, SILICOS-IT, and iLOGP.
Table 4 lists the solubility of the phytocompounds. A compound's water solubility and lipophilicity are highly linked (Ottaviani et al., 2010). Factors like temperature, pressure, and the type of solvent used can impact solubility. The saturation concentration measures the extent of solubility by identifying the point at which the concentration of the solute in the solution reaches equilibrium and no longer increases with more solute (Lachman et al., 1986). Water solubility is determined using the following approaches: Log S (ESOL), Log S (Ali), and Log S (SILICOS-IT). The ESOL and Ali models, both topological methods readily available in Swiss ADME, are frequently utilised to predict water solubility
Table 5 describes the pharmacokinetic properties. The term pharmacokinetics was first introduced by F. H. Dost in 1953 in his text, Der BliitspiegeI-Kinetic der Konzentrationsablaiife in der Frieslauffliissigkeit (Dost, 1953). The purpose of pharmacokinetics is to study the time course of drug and metabolite concentrations or amounts in biological fluids, tissues, and excreta, and also of pharmacological response, and to construct suitable models to interpret such data. In pharmacokinetics, the data are analysed using a mathematical representation of a part or the whole of an organism.
Table 6 enlists the drug likeliness of the phytocompounds i.e properties a compound should possess to be considered a viable candidate for development into a pharmaceutical drug. It encompasses Lipinski violations, Ghose violations, Veber violations, Egan violations, Muegge violations, Bioavailability Score.
Table 7 describes Medicinal chemistry properties of Phytocompound. It refers to the chemical characteristics and bioactive properties that determine its effectiveness as a therapeutic agent, its safety profile, and its potential for use in drug development.
V. PREDICTING COMPOUND TOXICITY
The prediction of compound toxicities is an important part of the drug design development process. Toxic doses are often given as LD50 values in mg/kg body weight. Toxicity classes are defined according to the globally harmonized system of classification of labelling of chemicals (GHS).]:
- Class I: fatal if swallowed (LD50 ≤ 5)
- Class II: fatal if swallowed (5 < LD50 ≤ 50)
- Class III: toxic if swallowed (50 < LD50 ≤ 300)
- Class IV: harmful if swallowed (300 < LD50 ≤ 2000)
- Class V: may be harmful if swallowed (2000 < LD50 ≤ 5000)
- Class VI: non-toxic (LD50 > 5000)
Below mentioned table is the organ toxicity report of a selected compound Withanolide Q.
|
Classification |
Target |
Shorthand |
Prediction |
Probability |
|
Organ toxicity |
Hepatotoxicity |
dili |
Inactive |
0.96 |
|
Organ toxicity |
Neurotoxicity |
neuro |
Inactive |
0.92 |
|
Organ toxicity |
Nephrotoxicity |
nephro |
Active |
0.5 |
|
Organ toxicity |
Respiratory toxicity |
respi |
Active |
0.76 |
|
Organ toxicity |
Cardiotoxicity |
cardio |
Active |
0.64 |
The Compound belongs to Class 2 toxicity with LD50: 7mg/kg i.e. it can be fatal if swallowed. The compound has Nephrotoxicity, Respiratory toxicity and Cardiotoxicity.
VI. OBSERVATION
The above-mentioned properties provide valuable insights into how the compounds behave within the body, such as their absorption, distribution, metabolism, and excretion (ADME). For example, compounds like Withanolide Q, Withanolide R, and Withanolide M are predicted to have high gastrointestinal (GI) absorption, indicating that they are likely to be well absorbed when taken orally. However, most of these compounds do not penetrate the blood-brain barrier (BBB), meaning they may not have direct effects on the central nervous system. Additionally, compounds like Withanolide D and Withanolide O show potential as P-glycoprotein (Pgp) substrates, which may influence their bioavailability and transport across cell membranes.
The table also includes information on whether these compounds inhibit specific cytochrome P450 (CYP) enzymes, which play a crucial role in drug metabolism. None of the studied compounds are predicted to inhibit CYP1A2, CYP2C19, CYP2C9, CYP2D6, or CYP3A4, which suggests they may have a relatively low potential for drug-drug interactions via these pathways.
Table 6 presents the drug-likeness properties of the phytocompounds of Withania somnifera. These properties are based on various rules such as Lipinski's Rule of Five, Veber's Rules, and others that predict the likelihood of a compound being orally bioavailable and suitable for drug development. According to the data, most of the compounds in Withania somnifera do not violate any major drug-likeness criteria, indicating that they possess characteristics typical of drug-like molecules. The bioavailability score for all the compounds is 0.55, which is considered moderate and suggests these compounds could have potential as therapeutics if further developed. Table 7 highlights the medicinal chemistry properties of the phytochemicals from Withania somnifera, including the PAINS (pan-assay interference compounds) and Brenk alerts, which are used to assess the likelihood of a compound interfering in biological assays or showing toxic effects. The synthetic accessibility is also provided, which indicates how easy or difficult it might be to synthesize these compounds. The compounds from Withania somnifera generally show moderate synthetic accessibility, suggesting they could be synthesized with a reasonable level of difficulty.
Conclusion
Withania somnifera (Ashwagandha) possesses a variety of bioactive compounds with potential therapeutic effects. These include anti-inflammatory, anti-cancer, anti-stress, and immunomodulatory properties. The phytochemicals such as Withanolide D, Withanolide M, Withaferin A, and others are well-studied for their potential in treating conditions like stress, depression, and cancer, offering promise for therapeutic application. The pharmacokinetic and physicochemical profiles indicate that these compounds are generally well-absorbed in the gastrointestinal tract, with moderate solubility and lipophilicity, which is important for their bioavailability. Their drug-likeness properties suggest that these compounds have the potential to be developed further as therapeutic agents, though additional studies, including clinical trials, are required to assess their safety, efficacy, and clinical application.
References
[1] https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=2bdff82eb23a373885252c87b53135b2fc9adde4 [2] https://pubchem.ncbi.nlm.nih.gov/compound/161671#section=2D-Structure [3] https://www.sciencedirect.com/topics/chemistry/lipophilicity [4] file:///C:/Users/91630/Downloads/P_Tuberosa%20Paper%20(1).pdf [5] https://deepblue.lib.umich.edu/bitstream/handle/2027.42/24565/0000847.pdf?sequence=1 [6] file:///C:/Users/91630/Downloads/molecules-27-05055-v2.pdf [7] https://japsonline.com/admin/php/uploads/364_pdf.pdf [8] https://en.wikipedia.org/wiki/Druglikeness [9] Banerjee P., Kemmler E., Dunkel M., Preissner R.: ProTox 3.0: a webserver for the prediction of toxicity of chemicals Nucleic Acids Res (Web server issue 2024); NAR [10] Banerjee P., Eckert O.A., Schrey A.K., Preissner R.: ProTox-II: a webserver for the prediction of toxicity of chemicals.Nucleic Acids Res (Web server issue 2018); NAR
Copyright
Copyright © 2025 Krithi Sastry. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66870
Publish Date : 2025-02-07
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

