Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Pneumococcal Vacine: Overall View
Authors: Dr. G. Usha Kiran, B. Aasritha, B. Jhansi lakshmi, J. Lulika Kumari, B. Sujitha, G. Thanmaya swathi
DOI Link: https://doi.org/10.22214/ijraset.2024.63970
Certificate: View Certificate
Abstract
Streptococcus pneumonia is a leading cause of invasive pneumococcal disease (IPD), resulting in significant morbidity and mortality worldwide. Pneumococcal vaccines have been instrumental in preventing IPD, particularly in vulnerable populations such as children , older adults and those with compromised immune systems.This article reviews current landscape of pneumococcal vaccine , including the available conjugate and polysaccharide vaccines, their immunogencity,efficacy and safety profiles.We discuss the impact of pneumococcal vaccination on IPD incidence,stereo type distrubuttion and anti biotic resistance.Additionally, we explore emerging vaccine technologies,such as protein based and mRNA vaccines,and their potential to enhance protection against pneumococcal disease.The article concludes with a discussion on the future directions for pneumococcal vaccine development,including the need for broader protection , improved immunogencity and increased access to vaccines in low- resource settings.
Introduction
I. INTRODUCTION
Vaccines serve as vital tools in safeguarding individuals from severe and often fatal diseases by enhancing the body's natural defense mechanism, known as the immune system. They replicate the body's response to an invasion by pathogens, such as viruses or bacteria, without causing illness. Typically, vaccines consist of either inactivated or weakened forms of these pathogens, ensuring that they do not induce the diseases they are designed to prevent, nor do they pose any risk to children (1). A vaccine can be defined as either an inactivated or attenuated pathogen or a component thereof (such as nucleic acid or protein) that, when introduced into the body, triggers a protective immune response. This process enables the immune system to remember how to effectively combat these pathogens. Consequently, if the body encounters a similar but live and virulent pathogen in the future, the immune system can swiftly eliminate it, preventing illness. Thus, vaccines are both safe and effective in protecting vaccinated individuals and the broader community (2). According to the World Health Organization (WHO), current immunization programs save an estimated 2 to 3 million lives annually, significantly contributing to the decline in mortality rates among children under five years old, which decreased from 93 deaths per 1,000 live births in 1990 to 39 deaths per 1,000 live births in 2018 (3).
II. CLASSIFICATION OF VACCINES
Vaccines can be categorized based on their synthesis methods, which include the following types:
1) Live Attenuated Vaccines: These vaccines are created by weakening pathogens, such as viruses or bacteria, through genetic modifications to inhibit their growth, ensuring they do not cause disease in the host. Some live vaccines utilize a related organism that naturally has limited growth in humans. The attenuated pathogen elicits a comprehensive immune response akin to that produced by a natural infection. Examples include:
- Oral Sabin polio vaccine
- MRV Vaccine (Measles, Mumps, Rubella, and Varicella)
- Nasal influenza vaccine
- Bacille Calmette-Guerin (BCG) vaccine
- Varicella vaccine
- Rotavirus vaccine
2) Inactivated or Killed Vaccines: In this category, the pathogen responsible for the disease is either killed or inactivated, typically through high temperatures or chemical agents such as formalin. These vaccines, upon administration, provoke a strong immune response that closely resembles the reactions observed during an actual infection. Examples include:
- Typhoid vaccine
- Influenza vaccine
- Salk polio vaccine
- Hepatitis A vaccine
3) Acellular or Subunit Vaccines: Acellular vaccines do not contain whole cells; instead, they consist of specific polysaccharides or proteins derived from the surface of bacteria or viruses. These components are recognized by the immune system as foreign, prompting an immune response. There are various types of acellular vaccines:
4) Toxoid Vaccines: Certain pathogenic bacteria produce toxins or harmful proteins during infection. Toxoid vaccines are developed by chemically inactivating these toxins, rendering them non-toxic while still eliciting a strong immune response. Examples include:
- Diphtheria vaccine
- Tetanus vaccine
- Pertussis vaccine
5) Conjugate Vaccine: Historically, polysaccharide vaccines were developed using sugar molecules found on the surface of bacteria; however, their efficacy in infants and young children was found to be limited. Researchers identified that these vaccines could achieve improved effectiveness when the bacterial polysaccharide molecules are chemically bonded or conjugated to a carrier protein. The incorporation of additional proteins imparts the immunological properties of the carrier to the antigen, thereby eliciting a more robust immune response that is sufficient for younger populations as well. Examples include:
- Hemophilus influenza type b (Hib) conjugate vaccine
- Pneumococcal conjugate vaccine
- Meningococcal C conjugate vaccine.
6) Recombinant Vaccine: A fragment of DNA is extracted from the pathogenic bacterium or virus. This specific gene is then integrated into a plasmid or carrier vehicle, facilitating the production of substantial quantities of precisely defined proteins, which are subsequently utilized as vaccines. Examples:
- Hepatitis B vaccine
- Human papilloma virus (HPV) vaccine.
7) DNA/RNA Vaccine: Genetic material, either in the form of DNA or RNA, from the pathogenic bacterium or virus is introduced into human cells, prompting the cellular machinery to synthesize the protein encoded by the inserted gene(s) of the pathogen. The immune system recognizes this protein as foreign, triggering an immune response against the entire pathogen. Currently, various types of nucleic-acid vaccines are undergoing development, pre-clinical, and clinical evaluation phases, such as the HIV vaccine.
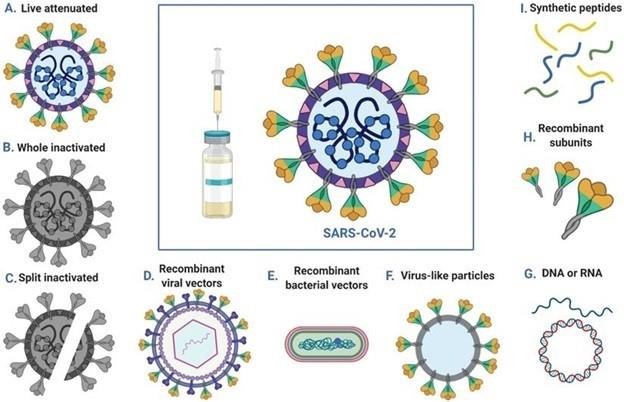
III. PATHOPHYSIOLOGY
Microorganisms are omnipresent, existing both in our surroundings and within our own bodies. When an individual is vulnerable and comes into contact with a pathogenic organism, it may result in illness or even fatality. The human body possesses various mechanisms to protect itself against pathogens, which are organisms that cause disease. Physical barriers such as skin, mucus, and cilia—tiny hair-like structures that help clear debris from the lungs—function to block pathogens from entering the body initially. In instances where a pathogen does invade, the immune system is activated, launching an attack to eliminate or neutralize the intruder.
The body's innate response involves recognizing pathogens, which can be bacteria, viruses, parasites, or fungi that induce disease. Each pathogen comprises distinct components, often unique to the specific organism and the illness it triggers.
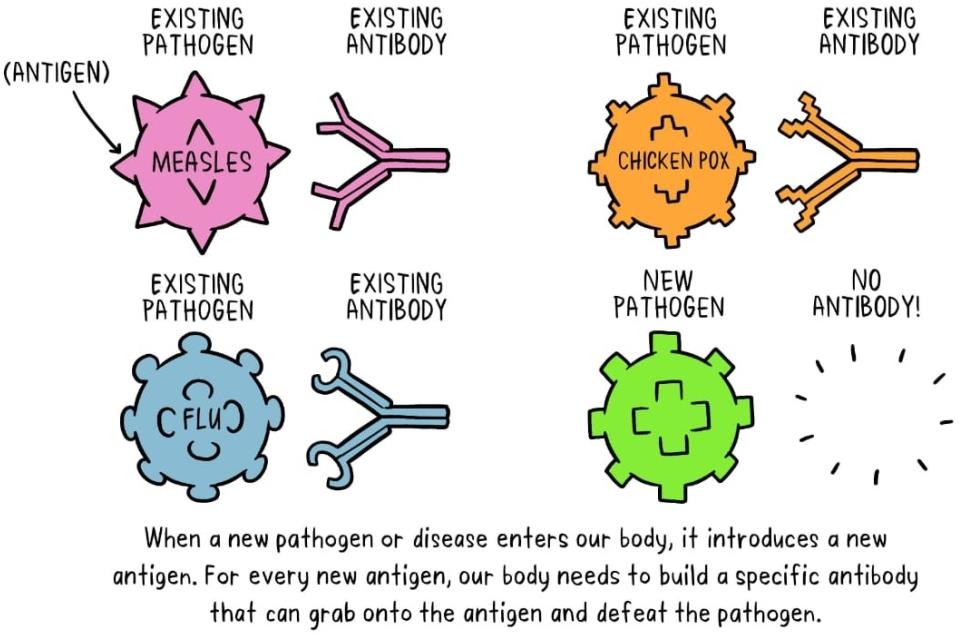
The component responsible for eliciting the production of antibodies is known as an antigen. The antibodies generated in response to the antigen play a crucial role in the immune response. One can liken antibodies to soldiers in the body’s defense mechanism, with each antibody trained to identify a particular antigen. The human body contains a vast array of antibodies. Upon first exposure to an antigen, the immune system requires time to react and generate the specific antibodies needed to combat that antigen.
During this period, the individual is at risk of falling ill. Once the antigen-specific antibodies are generated, they collaborate with the immune system to eliminate the pathogen and halt the progression of the disease. Typically, antibodies produced for one pathogen do not confer protection against another, unless the pathogens are closely related, akin to cousins. Following the initial immune response to an antigen, the body also develops memory cells that produce antibodies, which persist even after the pathogen has been eradicated.
If the individual encounters the same pathogen again, the immune response is significantly quicker and more efficient than during the initial exposure, as the memory cells are primed to produce antibodies against that specific antigen. This indicates that if the individual encounters the harmful pathogen in the future, their immune system will be prepared to respond promptly, thereby safeguarding against illness.
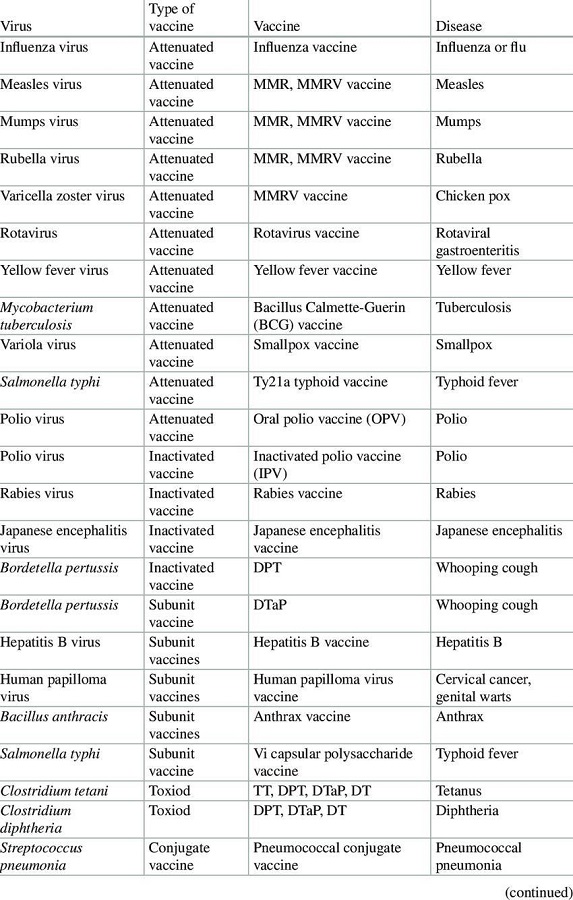
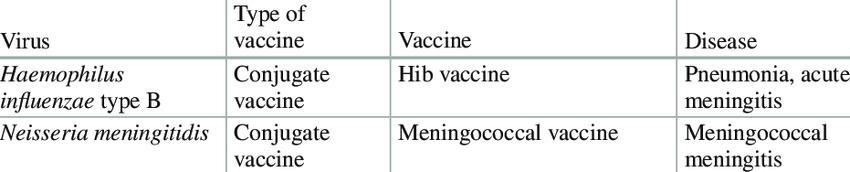
IV. PNEUMOCOCCAL VACCINE
Pneumococcal vaccine is a type of vaccine that protects against pneumococcal disease, which is caused by the bacterium Streptococcus pneumonia (also known as pneumococcus).
In developed countries, pneumococcal infection is responsible for approximately 30% of all adult pneumonia cases and has a mortality rate of 11% to 40% [1] The vaccine helps prevent:
- Pneumonia (infection of the lungs)
- Meningitis (infection of the lining around the brain and spinal cord)
- Sepsis (blood infection)
- Otitis media (middle ear infection)
- Sinusitis (sinus infection)
|
RISK ASSESSMENT OF PNEUMOCOCCAL DISEASES |
|
|
Immunocompetent individuals with high-risk comorbidities |
Chronic heart disease (with exception of isolated hypertension) Chronic lung disease Diabetes mellitus Chronic liver disease
|
|
Functional or anatomical asplenia |
Sickle cell disease and other hemoglobinopathies Congenital on acquired asplenia/splenic dysfunction
|
|
Immunosuppressed individuals |
HIV Chronic renal failure |
V. TYPES OF PNEUMOCOCCAL VACCINES
There are two primary categories of pneumococcal vaccines:
- PCV (Pneumococcal Conjugate Vaccine): Protects against 13 types of pneumococcal bacteria (PCV13) or 20 types (PCV20).
- PPSV (Pneumococcal Polysaccharide Vaccine): Protects against 23 varieties of pneumococcal bacteria.
The pneumococcal vaccine is recommended for:
- Children under 2 years old
- Adults 65 years and older
- People with certain medical conditions (e.g., heart disease, diabetes, chronic obstructive pulmonary disease)
- People with weakened immune systems (e.g., HIV/AIDS, cancer)
The vaccine is typically administered through injection, and the schedule may vary depending on age, health status, and other factors.
VI. MECHANISM OF ACTION OF PNEUMOCOCCAL VACCINE
Polysaccharide antibodies have been accessible since the mid1980s. These antibodies contain decontaminated capsular polysaccharides from 23 pneumococcal serotypes (1, 2, 3, 4, 5, 6b, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F and 33F). Polysaccharides basically actuate a B-cell-dependent resistant reaction by means of discharge of immunoglobulin M (IgM) [8]. Such polysaccharide antibodies are not suggested for utilize in children <2 a long time of age, likely due to their youthful resistant framework. Immunization of grown-ups with polysaccharide immunizations requires re-vaccination after 5–6 a long time [9]. However, non-responders to inoculation are particularly visit in more seasoned patients [10]. It has too been watched that antibodies against bacterial capsular polysaccharides are troublesome to actuate in reaction to non-conjugated polysaccharide antibodies for comparing meningococcal and Hemophilus antibodies [11,12].
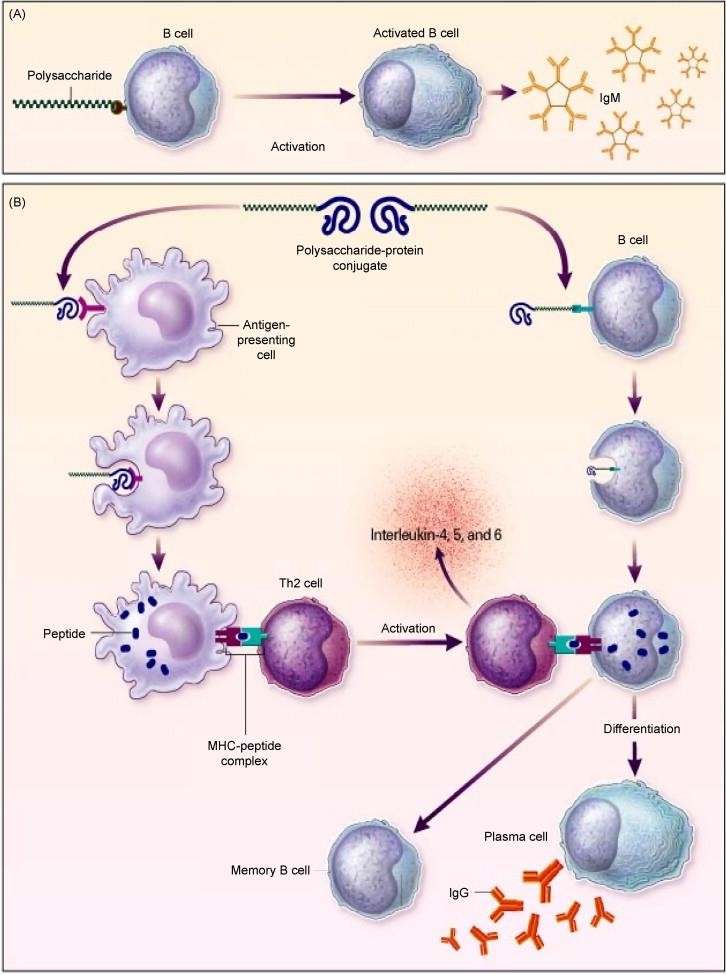
The heptavalent pneumococcal conjugated immunization (PCV-7) contains capsular polysaccharides from those pneumococci (4, 6B, 9V, 14, 18C, 19F and 23F) that are most as often as possible included in pediatric diseases. In the PCV9 RCT, when investigation was stratified by human immunodeficiency infection (HIV) status, VE was by and large as it were watched in HIV-uninfected children, with the special case of hMPV [13, 14, 15]. Capsular polysaccharides of PCV-7 are conjugated to exceedingly immunogenic cross-reactive fabric 197 (CRM197), a non-toxic diphtheria toxoid protein. Work of this pneumococcal immunization is especially effective in the inoculation of youthful children. Comparable to Hemophilus influenzae sort B conjugate inoculation, CRM197-specific sort 2 aide T (Th2) cells connected with B-cells that have bound and disguised the polysaccharide– CRM197 complex through polysaccharide-specific IgM and in this way display the prepared CRM197 protein along with MHC II to effector T-cells. This sort of versatile safe reaction is characterized by counter acting agent isotype exchanging and the era of memory B-cells.
Conclusion
The study emphasizes the efficacy of pneumococcal vaccinations in avoiding pneumococcal illnesses such as pneumonia, meningitis, and sepsis, especially in high-risk populations including young children, the elderly, and those with specific health problems or compromised immune systems. The two primary forms of pneumococcal vaccinations, PCV and PPSV, provide comprehensive coverage against pneumococcal serotypes, protecting against both common and harmful strains. As a result, widespread use of pneumococcal vaccinations has resulted in a large reduction in the prevalence of pneumococcal illnesses, so improving public health and averting serious and potentially fatal infections.
References
[1] Plotkin SA, Orenstein W, Offit PA. Vaccines. 6th ed. Philadelphia: Saunders; 2013. Pp. 1141–96. [Google Scholar] [2] Pollard, A. J., & Bijker, E. M. (2021). A guide to vaccinology: from basic principles to new developments. Nature Reviews Immunology, 21, 83–100. [3] World Health Organization. Child mortality and causes of death. WHO https://www.who.int/gho/child_health/mortality/mortality_under_five_text/en/ (2020). [4] A guide to vaccinology: from basic principles to new developments : Andrew J. Pollard & Else M. Bijker ,Nature Reviews Immunology volume 21, pages83–100 (2021). [5] India Science and Technology. (n.d.). Types of COVID-19 Vaccines. India Science and Technology. [6] 1. Kumar, V., Abbas, A. K., & Aster, J. C. (2020). Robbins and Cotran Pathologic Basis of Disease. Elsevier. (Chapter 1: The Cell and Molecular Biology of Disease) [7] Bridy-Pappas AE, Margolis MB, Center KJ, Isaacman DJ. Streptococcus pneumoniae: description of the pathogen, disease epidemiology, treatment, and prevention. Pharmacotherapy. 2005;25(9):1193–1212. [PubMed][Google Scholar] [8] A. de Roux et al. Immunogenity of the pneumococcal polysaccharide vaccine in COPD patients. The effect of systemic steroids Respir Med (2004) [9] R.R. Reinert et al. Molecular epidemiology of penicillin-non-susceptible Streptococcus pneumoniae isolates from children with invasive pneumococcal disease in Germany Clin Microbial Infect (2007) [10] T. Bauer et al. Streptococcus pneumoniae in community-acquired pneumonia. How important is drug resistance? Med Clin North Am (2001) [11] R.R. Reinert et al. Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001– 2003 Clin Microbial Infect (2005) [12] M.J. Fine et al. Validation of a pneumonia prognostic index using the Metagroups Comparative Hospital Database Am J Med (1993) [13] Madhi SA, Klugman KP; Vaccine Trialist Group. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med2004; 10:811–3. Google Scholar Crossref PubMed World Cat [14] Madhi SA, Ludewick H, Kuwanda L, et al. Pneumococcal coinfection with human metapneumovirus. J Infect Dis 2006; 193:1236–43. Google Scholar Crossref PubMed World Cat [15] Nunes MC, Cut land CL, Klugman KP, Madhi SA. Pneumococcal conjugate vaccine protection against coronavirus-associated pneumonia hospitalization in children living with and without HIV. mBio 2021; 12: e02347-20. Google Scholar Crossref PubMed \'World cat .
Copyright
Copyright © 2024 Dr. G. Usha Kiran, B. Aasritha, B. Jhansi lakshmi, J. Lulika Kumari, B. Sujitha, G. Thanmaya swathi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63970
Publish Date : 2024-08-13
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

