Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Comprehensive Review on Polymers Used in Insitu Gelling System
Authors: Ms. Rajeshwari G. Khairnar , Mr. Ganesh Verma , Mr. Ritesh Wani , Dr. Rupali R. Tasgaonkar
DOI Link: https://doi.org/10.22214/ijraset.2024.61900
Certificate: View Certificate
Abstract
In situ gelling formulations are drug delivery systems which typically exist in a liquid form at room temperature and change into gel state after application to the body in response to various stimuli such as changes in temperature, pH and ionic composition.[1] In situ gels have become one of the most prominent and accessible systems. These systems have several advantages like simple manufacturing, easy to use, improved adherence, and patient comfort by minimizing drug administration frequency by its unique characteristic features of sol to gel transition. In the \'sol-gel\' method, the precursor goes through hydrolysis and polymerization or condensation to produce a colloidal suspension or solution. As they can administer in solution form, these in situ gelling systems undergo gelation at the achievement site. Some researchers recently developed in situ gelling systems of liposomes, microspheres, nanoemulsions, nanospheres, etc. This review mainly focused on the introduction, advantages, disadvantages, types of polymers, and suitable characteristics for preparing in situ gels.[2]
Introduction
I. INTRODUCTION
In situ gelling systems are polymeric formulations that are in sol forms before entering in the body, but change to gel forms under the physiological conditions. The sol-gel transition depends on one or a combination of different stimuli, like pH change, temperature modulation, solvent exchange, ultra violet irradiation and the presence of specific ions or molecules. Drug delivery systems having such properties can be widely used for sustained delivery vehicle preparation of the bioactive molecules. Some important advantages of these smart systems are ease of application and reduced frequency of administration, as well as protection of drug from environmental condition changes[3]
II. OPHTHALMIC INSITU GEL
Ophthalmic in-situ gelling is comprising of environmentally sensitive polymers that will be altered structurally with the small changes in specific conditions like pH, temperature and ionic strength in the environment. In-situ forming gels are liquids during instillation into the eye and then undergoes rapid gelation in the cul-de-sac of the eye to form viscoelastic gels in response to environmental changes lastly release the drug slowly under physiological conditions[4]
III. POLYMERS OF INSITU GELLING SYSTEM
In situ forming polymeric formulations are drug delivery systems that are in sol form before administration in the body, but once administered, undergo gelation in situ, to form a gel. The formation of gels depends on factors like temperature modulation, pH change, presence of ions and ultra violet irradiation, from which the drug gets released in a sustained and controlled manner. Various polymers that are used for the formulation of in situ gels include gellan gum, alginic acid, xyloglucan, pectin, chitosan, poly(DL-lactic acid), poly(DL-lactide-co-glycolide) and poly-caprolactone. The choice of solvents like water, dimethylsulphoxide, N-methyl pyrrolidone, triacetin and 2-pyrrolidone for these formulations depends on the solubility of polymer used. Mainly in situ gels are administered by oral, ocular, rectal, vaginal, injectable and intraperitoneal routes. The in situ gel forming polymeric formulations offer several advantages like sustained and prolonged action in comparison to conventional drug delivery systems. The article presents a detailed review of these types of polymeric systems, their evaluation, advancements and their commercial formulations. From a manufacturing point of view, the production of such devices is less complex and thus lowers the investment and manufacturing cost.[5]
IV. SUITABLE CHARACTERISTICS OF POLYMERS
An essential ingredient in the manufacture of any gel is a polymer. Some of the relevant polymer characteristics for in situ gels given below:
It should be compatible
It should influence tear behavior
It should not provide any toxic effects
It should have pseudo-plastic behavior
It should have good tolerance and optical clarity
It should be capable of adhering to the mucous membrane
It should be capable of declining the viscosity with a boost in shear rate.[6]
V. CLASSIFICATION OF IN SITU GEL POLYMERS
Based on their origin, polymers are classified or the mechanism of gelation. According to a source in situ, gelling systems classified into two types:
- Natural polymers (E. g., Alginic acid, Carrageenan, chitosan, Guar gum, gellan gum, pectin, sodium hyaluronate, xanthan gum, xyloglucan, etc.)
- Synthetic or semi-synthetic polymers (E. g., Cellulose acetate phthalate, hydroxypropyl methyl cellulose, polyacrylic acid, poly(lactic-co-glycolic acid, poloxamers).[7]
A. Natural Polymers
- Alginic Acid or Sodium Alginate: A biodegradable, hydrophilic, non-toxic, linear block copolymer polysaccharide consists of β-D-mannuronic acid and α-L-glucuronic acid residues joined by 1,4-glycosidic linkages. It is used as a vehicle for ophthalmic formulations. Alginate transforms into a stable gel upon exposure to divalent cations (Ca+2, Mg+2) by cross-linking the carboxylate groups, which is not easily eroded by tear fluid.[8]
- Carrageenan: It is used as a home remedy to cure a cold and cough as gelatine. Depending on the sulfate group number and position classified into three types:
a. Iota carrageenan: It forms an elastic gel in the presence of calcium or potassium ions and completely soluble in hot water.
b. Kappa carrageenan: It forms a 'gel' in the presence of potassium ions and shows similar properties of locust bean gum like, soluble in hot water.
c. Lambda carrageenan: It does not induce gel formation, but it forms highly viscous solutions and is completely soluble in cold water.[9]
3. Chitosan: It is a biodegradable, biocompatible, thermosensitive, pH-dependent, cationic, amino polysaccharide obtained by alkaline deacetylation of chitin. Gelling of chitosan occurs by pH and temperature changes. It has excellent mucoadhesive properties due to the electrostatic interaction between positively charged chitosan and negatively charged mucosal surfaces. At low critical solution temperatures due to extreme hydrophobic interactions, gels formed with electrostatic forces. At upper critical solution temperature, exhibiting polymers are used for the gelation process of chitosan. Due to availability, non-toxic, inexpensive, etc., this is the second most abundant polysaccharide using after cellulose.[10]
4. Guar Gum or Guaran: It is soluble in water but insoluble in hydrocarbons, fats, ester, alcohols, and ketones. It shows better dispersibility and forms high viscous colloidal solutions with hot and cold water with small amounts. Temperature changes cause a reversible shift in gel formation.[11]
5. Gellan Gum: It is commercially known as Gelrite or Kelcogel, and it is a linear, water-soluble, temperature-dependent, extracellular, hetero, anionic polysaccharide; like alginate, this gellan gum form gel in the presence of metal cations (mono or divalent). Monovalent cations such as Na+or K+and divalent cations such as Ca+2 or Mg+2 induce cross-linking gelation. The gelation includes the formation of double-helical junction zones followed by aggregation of the double-helical segment to form 3-D networks by complexation with cations and hydrogen bonding with water. In the preparation of in situ gels, it is one of the most commonly used polymers.[12]
6. Pectin: A family of cationic, linear polysaccharides comprises α-(1, 4)-D galacturonic acid residues. In the presence of H+ions, the gelation of pectin will occur, a source of mono, divalent, and trivalent ions. It is only applicable to water-soluble formulations and not for the organic solvents. Monovalent cations (alkali metal) salts of pectin and pectic acids are soluble in water. But di and trivalent cationic salts are weakly soluble or insoluble in water. When the addition of water to dry powdered pectin, clumps (i.e., semi-dry packets) formed due to its tendency to hydrate and solubilization of cluster's done by mixing with a water-soluble carrier. The degree of methylation (DM), defined as the percentage of carbonyl groups esterified with methanol. Based on the degree of esterification, pectins classified into two categories:
a. Low methoxy pectins; less than 50% of the carboxyl groups methylate the pectins.
b. High methoxy pectins; more than 50% of the carboxyl groups methylate the pectins.[13]
7. Sodium Hyaluronate: It is a water-soluble form of the sodium salt of hyaluronic acid. It is a natural, endogenous polysaccharide that supports producing collagen and maintains elasticity in the body. It also increases formulation stability and reduces the probability of oxidation.[14]
8. Xyloglucan or Tamarind Gum: Xyloglucan is an abundant, hemicellulosic polysaccharide due to the non-toxic, biocompatible, and biodegradable nature, potentially using in several delivery systems. It is partially degraded by β-galactosidase and undergoes gelation by the thermoresponsive process. When used in oral delivery shows gelation time up to minutes and allows gelation in the stomach in chilled condition. Like, poloxamer it exhibits gelation on heating/refrigerator temperature or cooling from higher heat. Xyloglucan has the gelling ability in the presence of sugars (40-65%) or alcohols over a wide pH range. Still, in the combination (20% alcohols), the sugars are substantially reduced to form a gel.[15]
B. Synthetic or Semi-Synthetic Polymers
- Cellulose Acetate Phthalate (CAP): CAP also knows as pseudo latex. It is artificial latex, prepared in an aqueous medium by dispersion of a pre-existing polymer. It is pH sensitive, cross-linked polyacrylic polymers with potentially useful properties for sustained drug delivery to the eye because latex is a free-running solution at a pH of 4.4, which undergoes coagulation tear fluid, raises the pH to pH 7.4. CAP is used to monitor the ocular residence time of an ophthalmic preparation in γ-scintigraphy, and the production doesn't require the use of organic solvents.[16]
- Hydroxypropyl methylcellulose (HPMC): This is a biocompatible, thermoreversible, mucoadhesive polymer. It is a type of cellulose ether due to high swellability, thermal gelation properties, and used as hydrophilic matrices and used for oral drug delivery systems. HPMC used in combination with carbopol, enhancing the solution's viscosity while reducing the solution'sJadi. HPMC goes for gelation at higher temperatures due to the interaction between hydrophobic components of the polymer. It was playing an active role in aqueous solution formation for topical treatment of the eye. It proved to be essential to formulate vaginal mucoadhesive film with CR of S-nitroso glutathione and effects on the gelling behavior.[17]
- Methylcellulose (MC): It is also a cellulose derivative, used as in situ gelling polymer. Several cellulose derivatives stay on liquid at low temperatures and become gel upon heating. For example, MC and HPMC's aqueous solution undergoes a phase transition into gels between 40-50 °C and 75-90 °C, respectively. However, MC and HPMC's phase transition temperature is higher than the physiological temperature but lowered by making chemical and physical changes in the polymers. Hydrophobic interaction among molecules with methoxy groups causes gelation of HPMC and MC solutions. Polymer-polymer contact occurs between macromolecules due to hydration at a lesser temperature. The hydration is lost gradually on increasing the heat consequential in lower viscosity. At the transition where enough dehydration of the polymers takes place, they start associating, and the thickness starts rising, showing a network structure formation. At low temperature (30 °C) solution is in liquid form, and when the temperature increased (40-50 °C) and gelation occurred.[18]
- Polyacrylic acid (PAA): PAA is commercially known to be carbopol. It is widely used in ophthalmology for enhancing pre-corneal retention. It can exhibit excellent mucoadhesive properties to compare with other cellulose derivatives. Comparing different grads such as carbopol 910, 934, 940, 941, etc. concluded that 940 showed superior one [77, 82-84]. Poly (lactic-co-glycolic acid) or PLGA It is a biocompatible and biodegradable polymer. It is a synthetic copolymer of polylactic acid (PLA) and polyglycolic acid (PGA). These systems are applied to controlled drug delivery and are available as implants, microparticles, and in situ implants in the market. PLGA is one of the most capable polymers used to fabricate drug delivery and tissue engineering applications because of its long clinical experience [85-87]. Poloxamers: Poloxamers are commercially known as pluronic and used in thermosensitive in situ gels. It has excellent thermal setting properties and increases drug residence time. It is a water-soluble tri-block copolymer and consists of two polyethylene oxide (PEO) and polypropylene oxide (PPO). Pluronic F127 is the most commonly used poloxamer polymer in pharmaceuticals due to its colorless and transparent gels forming character. It consists of PEO (70%) and PPO (30%). A copolymer pluronic F127-g-poly (acrylic acid) was used as in situ gelling vehicles to prolong the residence time and better bioavailability of the ocular drugs.[19]
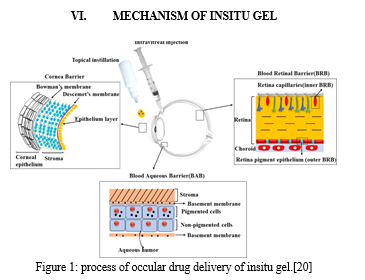
Conclusion
The use of biocompatible, biodegradable, and water-soluble polymers for the in situ gel formulation can make excellent and excellent drug delivery systems. In recent years, researchers have drawn interest, providing a lot of scope to advanced drug delivery techniques. A novel carrier can incorporate these systems to obtain sustained drug delivery in a much improved and extreme manner. Finally, in situ, gels are easy to apply and offer patient comfort and compliance.[21]
References
[1] Harish NM, Prabhu P, Charyulu RN, Gulzar MA, Subrahmanyam EV. Formulation and evaluation of in situ gels containing clotrimazole for oral candidiasis. Indian J Pharm Sci 2009;71:421-7. [2] Sonowal B, Deb P, Dash S. Studies on in-situ forming thermosensitive injectable polymeric gel for sustained drug delivery. Res J Pharm Technol 2017;10:1840-7. [3] Prasannan A, Tsai HC, Chen YS, Hsiue GH. A thermally triggered in situ hydrogel from poly (acrylic acid-co-N-isopropylacrylamide) for controlled release of anti-glaucoma drugs. J Mater Chem B 2014; 2: 1988-97. [4] Khan S, Patil K, Bobade N, Yeole P, Gaikwad R. Formulation of intranasal mucoadhesive temperature-mediated in situ gel containing ropinirole and evaluation of brain targeting efficiency in rats. J Drug Target 2010;18:223-34. [5] Jeong B, Kim SW, Bae YH. Thermosensitive sol–gel reversible hydrogels. Adv Drug Delivery Rev 2012;64:154-62. [6] Matyjaszewski K, Tsarevsky NV. Nanostructured functional materials prepared by atom transfer radical polymerization. Nature Chem 2009;1:276-88. [7] Gupta H, Velpandian T, Jain S. Ion-and pH-activated novel in-situ gel system for sustained ocular drug delivery. J Drug Target 2010;18:499-505. [8] Bai B, Zhou J, Yin M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet Explor Dev 2015;42:525-32. [9] Xin C, Lihong W, Qiuyuan L, Hongzhuo L. Injectable long-term control-released in situ gels of hydrochloric thiothixene for the treatment of schizophrenia: preparation, in vitro and in vivo evaluation. Int J Pharm 2014;469:23-30. [10] Packhaeuser CB, Schnieders J, Oster CG, Kissel T. In situ forming parenteral drug delivery systems: an overview. Eur J Pharm Biopharm 2004;58:445-55. [11] Hatefi A, Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Controlled Release 2002;80:9-28. [12] Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Delivery Rev 2009;61:158-71. [13] Rees CA, Provis JL, Lukey GC, Van Deventer JS. In situ ATR-FTIR study of the early stages of fly ash geopolymer gel formation. Langmuir 2007;23:9076-82. [14] Gong C, Shi S, Wu L, Gou M, Yin Q, Guo Q, et al. Biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PCL–PEG–PCL hydrogel. Part 2: sol-gel–sol transition and drug delivery behavior. Acta Biomater 2009;5:3358-70. [15] Shinde UP, Yeon B, Jeong B. Recent progress of in situ formed gels for biomedical applications. Progress in polymer science. Prog Polym Sci 2013;38:672-701. [16] Mundada AS, Avari JG. In situ gelling polymers in ocular drug delivery systems: a review. Crit Rev Ther Drug Carrier Syst 2009;26:85-118. [17] Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61-73. [18] Patil S, Kadam A, Bandgar S, Patil S. Formulation and evaluation of an in situ gel for ocular drug delivery of anticonjunctival drug. Cellul Chem Technol 2015;49:35-40. [19] Saini R, Saini S, Singh G, Banerjee A, Railmajra DS. In situ gels-a new trends in ophthalmic drug delivery systems. Int J Pharm Sci Res 2015;6:386-90. [20] Mahajan HS, Tyagi VK, Patil RR, Dusunge SB. Thiolated xyloglucan: synthesis, characterization and evaluation as mucoadhesive in situ gelling agent. Carbohydr Polym 2013;91:618-25. [21] Gupta S, Samanta MK, Raichur AM. Dual-drug delivery system based on in situ gel-forming nanosuspension of forskolin to enhance antiglaucoma efficacy. AAPS PharmSciTech 2010; 11:322-35. [22] Chang CF, Wang SC, Shigeto S. In situ ultra low-frequency raman tracking of the polymorphic transformation of crystalline 1, 1?-binaphthyl. J Phys Chem C 2014;118:2702-9. [23] Bashir A, Manzoor T, Malik LA, Qureashi A, Pandith AH. Enhanced and selective adsorption of Zn (II), Pb (II), Cd (II), and Hg (II) Ions by a dumbbell-and flower-shaped potato starch phosphate polymer: a combined experimental and DFT calculation study. ACS Omega 2020;5:4853-67. [24] Raizada A, Bandari A, Kumar B. Polymers in drug delivery: a review. Int J Pharm Pharm Res Dev 2010;2:9-20. [25] Boateng J, Okeke O, Khan S. Polysaccharide based formulations for mucosal drug delivery: a review. Curr Pharm Des 2015;21:4798-821. [26] Tinu TS, Litha T, Kumar Anil B. Polymers used in ophthalmic in situ gelling system. Int J Pharm Sci Rev Res 2013;20:176-83. [27] Mohanty D, Bakshi V, Simharaju N, Haque MA, Sahoo CK. A review on in-situ gel: a novel drug delivery system. Int J Pharm Sci Rev Res 2018;50:175-811. [28] Reddy K, Krishna Mohan G, Satla S, Gaikwad S. Natural polysaccharides: versatile excipients for controlled drug delivery systems. Asian J Pharma Sci 2011;6:275-86. [29] Li L, Ni R, Shao Y, Mao S. Carrageenan and its applications in drug delivery. Carbohydr Polym 2014;103:1-11.
Copyright
Copyright © 2024 Ms. Rajeshwari G. Khairnar , Mr. Ganesh Verma , Mr. Ritesh Wani , Dr. Rupali R. Tasgaonkar . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET61900
Publish Date : 2024-05-10
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

