Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Power Alcohol Production from Sawdust
Authors: Sunil Sable , Anubhav Shirke, Parag Bhadke, Suyash Burfule
DOI Link: https://doi.org/10.22214/ijraset.2025.66476
Certificate: View Certificate
Abstract
This research investigates the production of ethanol from waste sawdust generated during woodworking operations. Ethanol is a significant biofuel due to its use as an additive to gasoline and its applications as a solvent in both laboratory and industrial settings Ethanol has the potential to replace fossil fuels, reducing greenhouse gas emissions and air pollution. Additionally, its production can positively impact rural economies. Since ethanol is synthesized from biomass, which can be sourced from agricultural waste, this process not only supports sustainable energy practices but also creates job opportunities in rural areas where such waste is abundant. The production process involves several stages: Pretreatment (grinding the sawdust into a fine powder to enhance hydrolysis efficiency), Hydrolysis (alkaline hydrolysis with 0.1 N NaOH at 100°C and 1.5 atm pressure), Fermentation (using Saccharomyces cerevisiae), and Distillation. An autoclave is used during hydrolysis to ensure optimal pressure and temperature conditions. The research demonstrates that a substantial amount of ethanol can be produced from sawdust, which offers both environmental and economic benefits by recycling waste materials and creating a renewable energy source. Future research should focus on optimizing the pretreatment process to further enhance efficiency and explore other potential improvements in the production process.
Introduction
I. INTRODUCTION
The increasing demand for renewable energy sources, coupled with the need for effective waste management strategies, has led to the exploration of alternative methods for energy production. One promising avenue is the production of power alcohol from sawdust, which offers a sustainable solution by converting biomass resources into a valuable fuel source. This introduction provides an overview of power alcohol production from sawdust, highlighting its significance, benefits, and potential applications. Sawdust, such as crop residues, straw, husks, and bagasse, is generated in large quantities worldwide as a by-product of farming and food production. Traditionally, these waste materials were either left to decompose, causing environmental concerns, or were burned, leading to air pollution. However, with advancements in technology and growing awareness of sustainability, sawdust is now seen as a valuable resource that can be utilized to meet energy demands. Power alcohol, also known as ethanol, has emerged as a prominent candidate for renewable energy due to its numerous advantages. Ethanol stands out as a versatile fuel option capable of substituting for fossil fuels, thereby decreasing reliance on exhaustible resources and addressing greenhouse gas emissions. It burns cleaner than gasoline, resulting in lower emissions of harmful pollutants. Moreover, ethanol is easily accessible and can be generated from a diverse array of feedstocks, including agricultural residue.
II. LITERATURE REVIEW
W. Braide Et Al in his paper [1] gives us the knowledge of ethanol production from agro waste which includes Sugar bagasse, sugar cane bark, corn cob, corn stalk, and corn husk. First, it is hydrolysed, then it is fermented by using yeast. For hydrolysis autoclave equipment is used. Fermentation is done using saccharomyces cerevisiae for Five days. This fermentation is anaerobic fermentation. In their publication [2], R. Maryana et al. detailed that the process of deriving ethanol from bagasse involves three primary stages: pre-treatment, saccharification, and fermentation. First, the bagasse is washed, dried, and ground into powder. For pretreatment, alkali NaOH is used for hydrolysis, solid to liquid ratio is 1:12. pre-treatment damages the crystalline structure and lignin of bagasse which is important for further processes. There are different methods present including steam explosion, thermochemical pre-treatment, etc. The fermentation is done by using Saccharomyces cerevisiae. The sample is then filtered to get ethanol. M. H. Nasution Et Al in the paper [3] provides an overview of baggage containment measures. The steps involved in making bioethanol from sawdust are as follows: pre-treatment, hydrolysis using enzymes, fermentation, distillation, and dehydration. Approximately 3:4:3 ratios of lignin, cellulose and hemicellulose makes up the majority of sawdust. In order to facilitate the hydrolysis of cellulose, reduce its crystalline structure, and extract lignin and hemicellulose, a pretreatment process is essential for sawdust. Pre-treatment procedures come in a variety of forms, such as (i) physical pretreatment (ii) acid pre-treatment (iii) alkaline pre-treatment, which dissolves lignin, silica, and hemicelluloses to dissolve the cell wall; (iv) Organosolv pre-treatment; pre-treatment using organic solvents, through partial hydrolysis of lignin linkages, organosolv pretreatment effectively eliminates lignin from lignocellulosic materials. (v) Steam explosion; This hydrothermal pre-treatment method quickly decompresses the system and destroys the fibril structure as well as. (vi) moist oxidation. Wet oxidation refers to the procedure of subjecting biomass to temperatures exceeding 120°C, accompanied by the presence of water, oxygen, or air. Hemicelluloses can become more soluble through wet oxidation. Kongchamnan P. Et Al in the paper [4] investigated Liquid hot water (LHW) in this study as a pre-treatment method to improve the digestibility of pre-treated material for subsequent conversion into bioethanol. The maximum glucose yield was achieved at the optimal conditions of 160 C for 60 min with 0.050 M acid content (96.86%). A mixture comprising 1 g of natural sawdust and 15 mL of water was employed for the pre-treatment process. This involved altering concentrations of the H2SO4 acid catalyst (ranging from 0.025 M to 0.075 M), temperatures (ranging from 140°C to 180°C), and residence periods (spanning 30 to 90 minutes) Shaibani N. Et Al in his paper [5] explains that prior to enzymatic hydrolysis, biomass underwent alkali pre-treatment. When NaOH, KOH, and Ca(OH)2 were compared, it became clear that NaOH had a greater impact on the structure of bagasse. The four main phases in the manufacturing of ethanol from LB (lignocellulosic biomass) are pre-treatment, hydrolysis, fermentation, and recovery and purification. Aspergillus Niger, Trichoderma reesei, and Trichoderma longibrachiatum are the three most popular fungus used to make cellulose worked better to hydrolyzed cellulose and hemicellulose with A. Niger enzyme solution produce cellulase, cellulose and hemicellulose hydrolysis using A. Niger enzyme solution was better.
III. EXPERIMENTAL WORK
The project aims to extract ethanol from sawdust through a series of processes, including hydrolysis, fermentation, filtration, and distillation. The following methodology outlines the steps involved in each process:
A. Hydrolysis
250 grams of sawdust is taken and subjected to hydrolysis. The bagasse is mixed with 3 liters of 0.1 Normal NaOH in an autoclave reactor. The mixture is heated to a temperature of 100 degrees Celsius and maintained at a pressure of 1.5 atm for 45 minutes. This hydrolysis process breaks down the complex polysaccharides present in bagasse into simpler sugars.

Fig.1 Hydrolysis reactor
B. Fermentation
After hydrolysis, the resulting hydrolysate is cooled and transferred to a fermentation vessel. Brewer’s yeast, typically Saccharomyces cerevisiae, is added to the hydrolysate. The yeast consumes the sugars present in the hydrolysate, primarily glucose, and converts them into ethanol and carbon dioxide through the process of alcoholic fermentation. The fermentation process is allowed to proceed for a duration of 5 days under suitable conditions of temperature, pH, and nutrient availability.

Fig.2 During fermentation
C. Filtration
Once fermentation is complete, the fermented sawdust, along with any solid particles and strings, is subjected to filtration. A filtration system, such as a filter press or a filtration unit with appropriate filters, is set up. The fermented mixture is loaded into the system, and the solid particles and strings are retained by the filter medium, forming a filter cake. The liquid portion, containing ethanol and other dissolved components, passes through the filter medium and is collected as the filtrate

Fig. 3 Filtration
D. Distillation
The collected filtrate, which contains ethanol along with water and impurities, is subjected to distillation for the extraction and purification of ethanol. A distillation apparatus, such as a distillation column, is set up. The filtrate is heated in a boiling flask, and the vapors rise through a fractionating column. The ethanol, having a lower boiling point than water, vaporizes more readily and is condensed in a separate vessel. Multiple distillation cycles may be performed to increase the purity of ethanol.
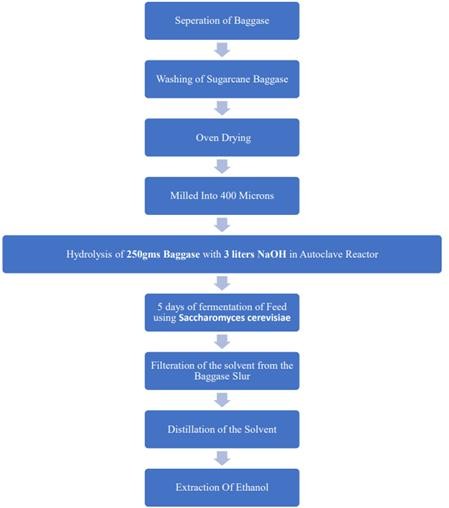
Fig. 4 Overall flow diagram
IV. RESULT AND DISCUSSION
The study successfully demonstrated the conversion of sawdust into ethanol, a renewable energy source. Hydrolysis of sawdust using NaOH effectively broke down complex polysaccharides into simpler sugars. Fermentation of the hydrolysate using Saccharomyces cerevisiae yielded ethanol and carbon dioxide over a 5-day period. Filtration removed solid particles, preparing the mixture for distillation. Distillation efficiently extracted and purified ethanol from the mixture. The study confirmed the potential of using alkali pre-treatment to enhance lignocellulosic breakdown. These findings highlighted the feasibility of converting sawdust into ethanol, contributing to renewable energy production and waste management strategies. In this experiment, we have used a NaOH concentration of 2%, which is lower than the concentrations used in some other studies. This reduces the cost and environmental impact of the pre-treatment process. It uses a fermentation time of 5 days, which is shorter than the fermentation times used in some other studies. This reduces the overall production time and cost. It achieves a higher ethanol yield than some other studies. This is likely due to the effectiveness of the alkali pre-treatment process and the optimization of the fermentation and distillation process.
A. Comparison
The yield of ethanol production from material depends on several factors including the composition of the material, the processing technology employed and the efficiency of the production process. but the main and common processes for all the materials are pretreatment, enzymatic hydrolysis, fermentation, separation, dehydration. The yield of ethanol varies with the technologies involved in above steps. Here is the comparison of substances and their ethanol production yield. 1. Sugar cane: - 389.22 ml per 1 liter of bagasse slurry. 2. Corn: - 1 liter per 2.8 kg of corn bagasse. 3. Wheat straw: - 5.4 kg dry raw material is needed to produce 1 L of ethanol. 4. Sawdust: - 10 kg of sawdust gave 500 cm 3 of ethanol.
Conclusion
The usage of sawdust as a feedstock for ethanol production offers a promising solution to address environmental concerns, promote energy sustainability, and foster rural development. The conversion of sawdust into ethanol provides several benefits, including reducing greenhouse gas emissions, minimizing waste disposal, and diversifying the energy mix. It also creates economic opportunities, particularly in agricultural regions, and contributes to the establishment of a circular economy by valorizing waste materials. Technological advancements have improved the efficiency and feasibility of the conversion processes, enabling better utilization of various types of sawdust.
References
[1] Braide (Dr), Wesley & Kanu, IA & Solomon, Oranusi&Adeleye, Samuel. (2016). Production of bioethanol from sawdust. Journal of Fundamental and Applied Sciences. 8. 372. 10.4314/jfas.v8i2.14. [2] [Hernawan, R. Maryana et al. \"Bioethanol production from sawdust by simultaneous saccharification and fermentation using Saccharomyces cerevisiae.\" AIP Conference Proceedings, 1823 (1), 020026 (17 March 2017). [3] M. H. Nasution et al. \"A review of sawdust pretreatment for bioethanol production.\" IOP Conference Series: Earth and Environmental Science, 963, 012014 (2022). DOI: 10.1088/1755- 1315/963/1/012014. [4] Khongchamnan, P. et al. \"Optimization of Liquid Hot Water Pretreatment and Fermentation for Ethanol Production from Sawdust Using Saccharomyces cerevisiae.\" Catalysts, 12, 463 (2022). DOI: 10.3390/catal12050463. [5] Shaibani, N. et al. \"Ethanol production from sawdust by means of on-site produced and commercial enzymes; a comparative study.\" Periodica Polytechnica Chemical Engineering, 56(2), 91–96 (2012). DOI: 10.3311/pp.ch.2012- 2.07. [6] Zhao, Y. et al. \"The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens.\" Food Chemistry, 185, 112-118 (2015). [7] Santos, VEN. et al. \"Chemicals, electricity and fuels from 28 biorefineries processing Brazil\'s sawdust: Production recipes and minimum selling prices.\" Renewable and Sustainable Energy Reviews, 53, 1443-1458 (2016). [8] Kumari, S. and Das, D. \"Improvement of gaseous energy recovery from sawdust by dark fermentation followed by biomethanation process.\" Bioresource Technology, 194, 354-363 (2015). [9] Li, Z. and Ge, Y. \"Antioxidant activities of lignin extracted from sawdust via different chemical procedures.\" International Journal of Biological Macromolecules, 51, 1116-1120 (2012). [10] Sukyai, P., Bezerra, T. L., and Ragauskas, A. J. \"A review of sawdust for second-generation bioethanol and biopower production.\" Biofuels, Bioproducts and Biorefining, 10(5), 634-647 (2016). [11] Conag, A. T. et al. \"Energy densification of sawdust through torrefaction under minimized oxidative atmosphere.\" Journal of Environmental Chemical Engineering, 5(6), 5411-5419 (2017).
Copyright
Copyright © 2025 Sunil Sable , Anubhav Shirke, Parag Bhadke, Suyash Burfule. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66476
Publish Date : 2025-01-11
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

