Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Pre-Clinical Requirements for Drug-Eluting Stents for CE Certification
Authors: Shailaja P., Snehalatha. G, Revathi. K, Likhita. P
DOI Link: https://doi.org/10.22214/ijraset.2023.50658
Certificate: View Certificate
Abstract
Drug Eluting Stents (DES) are small, expandable tubes that are implanted into a diseased, blocked peripheral or coronary artery through angioplasty to widen and increase the blood flow by slowly releasing the drug from device. They are covered in a medication that stops scar tissue from expanding upon that artery. In medical practice DES are frequently used for vascular disorders, exhibiting adequate potency and suitability within the acceptable range. Medical device regulations in European Union (EU) are covered by Directives 93/42/EEC, 98/79/EEC and 90/385/EEC which were recently replaced by new regulations (EU) 2017/745 on medical devices and (EU) 2017/746 on In vitro diagnostic medical devices. Across all the EU legislation, “CE Mark” or “Certificate of Compliance” has become a recognised term. The Competent authority’s (CA) certifies that a product conforms to required standards of EU, then the manufacturer display the “CE mark” on the label. The Drug-Device combination products like DES are included in Class-III high risk active implantable medical devices. The pre-clinical investigations of drug eluting stents often follow International Organization for Standardization (ISO) requirements, these vascular stents are covered under ISO 25539-2: 2020 - Cardiovascular Implants - Endovascular Devices, ISO 10993:2018- Biological Evaluation of Medical Devices and ISO 10555-1: 2013 – Intravascular catheters, sterile and single use medical devices.
Introduction
I. INTRODUCTION
Drug Eluting Stent (DES) consist both device and the drug with dual action it is also known as Combination Product. DES prevents coronary artery from narrowing again after angioplasty. DES is a metallic prosthesis (strut) implanted into the arterial wall and coated with a thin layer of biocompatible polymeric gel that encapsulates a therapeutic drug (coating) [1]. In order to unblock a tiny blocked segment of the coronary artery and enhance blood flow, stents are small, inflatable tubes that are implanted during angioplasty [2]. A manufacturer must abide to the requirements of the directive 2007/47/EC, Regulation (EU) 2017/745 and (EU) 2017/746 to lawfully market a medical device (MD) in EU [3, 4]. Medical device regulations in European Union (EU) are covered by Directives 93/42/EEC on medical device, 98/79/EEC on In vitro diagnostic medical devices and 90/385/EEC on Active Implantable Medical Devices which were recently replaced by new regulations (EU) 2017/745 on medical devices and (EU) 2017/746 on In vitro diagnostic medical devices [5].
The first endovascular treatment Percutaneous Transluminal Angioplasty (PTA) in 1964, which consists balloon dilation of the artery, has been widely accepted as a treatment mode for coronary disorders [6]. After successful PTA in the coronary arteries of the majority of the patients, quickly noticed that the vessels had restenosis after six months of follow-up. This problem was mostly brought on by negative remodeling, or contraction of the vessel wall. Later, metallic stents were developed to prevent this adverse consequence by maintaining the channel open mechanically. Although initial hyperplasia was not prevented by the metallic stents, negative remodeling was inhibited. Another breakthrough in endovascular therapy is the use of DES, which can mechanically diminish negative re-modeling and the drug can reduce the initial hyperplasia. Drug-eluting biodegradable stents have drawn the most recent attention; they perform their intended function before degrading, without leaving any foreign elements in the body.
II. MEDICAL DEVICE REGULATORY AUTHORITIES IN EUROPE
There are concerned authority for each member state from the European Union (EU) and its associate nations. The European Commission (EC) inspects medical devices before they have been sold within EU. The following are the EU regulatory bodies:
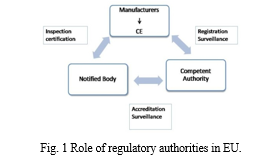
- Competent Authority (CA): An organization behind the member states to monitor the compliance with the national regulations and implement the Medical Device Directive's in state legislation [7].
- Notified Body (NB): A Notified body is a company chosen by an EU nation to examine a product's conformance before it is put on the market. Clinical trials are regulated by CAs, whereas NB is responsible for CE marking.
- Authorized Representatives: Any legal person within the EU who has received and accepted a written mandate from manufacturer, located outside the Union, to act on behalf of manufacturer in specific tasks relate to EU regulations. The role of medical device Manufacturer, Notified Body and Competent Authority in EU are depicted in Fig. 1.
III. DETERMINATION OF THE REGULATORY STRATEGY
It may be difficult to determine the most appropriate regulatory strategy for a combination product like DES. First, it's essential to think about how a medical devices and a drug are recognized in European regulations. According to European Union Medical Device regulations, a “Medical Device” means any Instrument, apparatus, software, implant, reagent, material or other article intended by the manufacturer to be used alone or in combination for diagnosis, prevention, treatment or alleviation of disease, replacement or modification of a physiological process and control of conception [8].
A. Classification of Medical Devices
Medical devices are categorized upon their intended use and inherent risks. They fall under the following categories as shown in Table 1.
|
CLASS |
RISK |
EXAMPLES |
|
Class I (Non-Measuring/non-Sterile) |
Low risk devices |
Urine Collection Bottles, Compression hosiery, plaster of Paris, cervical collars |
|
Class I (measuring/Sterile)
|
Low/medium risk devices |
Stethoscopes for diagnosis, conductive gels, eye occlusion plasters |
|
Class IIa |
Medium Risk & Short term invasive devices |
Tracheal tubes &lancets |
|
Class IIb |
Higher risk & Long-term invasive devices |
Surgical lasers |
|
Class III |
Highest Risk & including all active implantable devices |
heart-valves & Drug Eluting Stents |
Table 1: Risk based classification of medical devices.
A medical device containing a therapeutic agent as a secondary function like Drug-Eluting stents, are classified as high risk class-III devices by the European Regulatory authorities in accordance with Annex IX, Rule 13 of the MDD [9].
IV. COMPONENTS AND GENERATIONS OF DRUG ELUTING STENTS
The Drug-eluting stents have three main components:
- The Stent: Stents serve as mechanical scaffold for DES which can be either biodegradable or bio-stable metals. Metals make up the majority of bio-stable materials, e.g. 316L stainless steel, nitinol, titanium, platinum-iridium alloy, tantalum and cobalt-chrome alloy.
- The Coating material: Most coatings are made of polymers. There are many other coating options, but the polymeric coating with the drug is the most popular.
- The Drug: The drugs' primary functions are to increase neo-endothelialization, limit initial hyperplasia, and reduce the likelihood of thrombus development. For medications with a broad therapeutic window, a hydrophobic nature is preferred as these compounds may easily permeate through the artery walls in large quantities. Despite the fact that many drugs have been studied, they may typically be categorized into four categories:
a. Immune suppressants like sirolimus, tacrolimus and Everolimus.
b. Anti-proliferative agents eg: paclitaxel and its derivatives.
c. Anti-inflammatory agents eg: glucocorticoids and dexamethasone.
d. Pro-healing agents like HMG-CoA reductase inhibitors.
The Cypher® stent, which launched as the first DES, later Taxus® stent in 2004.The most recent advances in DES technology use stents made of biodegradable polymers, which degrade when they have completed distributing the medicine. There are three generations of Drug eluting stents as shown in Table 2:
|
PARAMETER |
Ist GENERATION DES |
IInd GENERATION DES |
IIIrd GENERATION DES |
|
Stent |
Cypher & Taxus |
Endevour & Xience |
BioMatrix FlexTM, NevoTM |
|
Drug |
Sirolimus & Paclitaxel [10] |
Zotarolimus & Everolimus |
Biolimus, Sirolimus & Everolimus |
|
Platform |
Stainless steel |
Cobalt chromium, thin strut stents |
Cobalt chromium, nickel- titanium |
|
Polymer |
Durable |
Persistent |
Bioabsorbable Polymer coated DES |
|
Risk |
Late stent thrombosis |
Stent exhibits clearly lower thrombosis as compared to first generation DES |
Improvement over DES |
Table 2: Generations of Drug Eluting Stents and their parameters.
V. PRE-CLINICAL TESTING OF DES
It is necessary to carry out pre-clinical and clinical investigations to show the MDs' efficacy and safety. Pre-clinical testing that complies with ISO standards makes proper recommendations for evaluating the safety and performance of the medical device. ISO standards required for preclinical studies of DES are as follows:
- ISO 25539:2012 - Cardiovascular Implants- Endovascular Devices – Part-2: Vascular Stents [11]
- ISO 10993 - Standard- Biological evaluation of medical devices [12]
- ISO 10993-1 - Evaluation and testing in the risk management process
- ISO 10993-3 - Tests for genotoxicity, carcinogenicity and reproductive toxicity
- ISO 10993-6 - Tests for local effects after implantation
- ISO 10993-9 - Framework for identification and quantification of potential degradation products
- ISO 10993-15 - Identification and quantification of degradation products from metals and alloys
- ISO 10555-1:2013 - Intravascular catheters- Sterile single use catheters-Part-1: General Requirements [13]
- ISO 10555-4:2013 - Intravascular Catheters-- Sterile and single-use catheters -- Part -4: Balloon dilatation catheters.
Following are the pre-clinical tests for DES in EU:
A. Bench Testing
Bench testing should be carried out on the metallic stent, if the stent strut’s surface is altered in to apply coating layer, additional testing may be necessary. It is crucial to consider the safety of the coated components, including the testing of the biological compounds and the polymeric carrier, while conducting bench test for non-resorbable DES. Unless otherwise justified, it should be done on final products. The parameters evaluated in bench test are shown in Table 3.
|
1. Stent Dimensional and Functional Attributes ?Dimensional and surface Area ?Stent Integrity ?Stent Longitudinal Strength , Recoil for Balloon Expandable Stents ?Radial Stiffness and Radial Strength , ?Mechanical Properties , Stress /Strain Analysis ?Accelerated Durability Testing ?Particulate Evaluation and coating durability (If coated) ?Magnetic Resonance Imaging (MRI) Safety and Compatibility |
|
2. Delivery System Dimensional and Functional Attributes ?Dimensional Verification, Delivery, Deployment, and Retraction ?Balloon Rated Burst Pressure ?Balloon Fatigue ?Balloon Compliance ?Balloon Inflation and Deflation Time ?Catheter Bond Strength, Tip Pull Test ?Flexibility and Kink Test, Torque Strength , Coating Integrity |
|
3.For Medical substance: ?Non-clinical Pharmacology and Toxicology ?Clinical Pharmacology ?Drug Release Kinetics ?Chemistry Manufacturing Controls (CMC) for the Medicinal Substance ?CMC for the Finished Product (includes the coating) |
|
4. For carrier ?Coating Characterization (i.e., chemistry, thickness and uniformity, adhesion to stent substrate), ?Coating Integrity, Fatigue Analysis, Corrosion properties and durability [14] ?Particulate Assessment ?Stability ?Characterization of degradation profile (if carrier is biodegradable) |
Table 3: List of Parameters evaluated during bench test.
B. Biocompatibility Testing
The term "biocompatibility" is used to explain how a DES that has been implanted interacts with the body. A biomaterial must affect the host environment as little as possible as one of its primary requirements. A biomaterial should have an active functional role in local tissue, should elicit a proper biological response, and the response to the biomaterial needs to be proper and will depend on the intended role. These three key principles have been incorporated into the definition of biocompatibility to reflect this realization [15].The choice of biomaterial is mainly 316L stainless steel, titanium and cobalt chromium alloys used extensively in clinical and vascular applications are highly corrosion resistant [16]. The ISO standards should be followed when conducting the biocompatibility testing.
The biological tests include: Cytotoxicity test, Delayed-type hypersensitivity, Intra cutaneous reactivity test, Acute toxicity, Sub acute and sub chronic toxicity, Genotoxicity, Implantation tests, Haemocompatibility test, Carcinogenicity, Reproductive and developmental toxicity, Biodegradation tests, Toxicokinetic studies, Immunotoxicology.
C. In vivo Testing
Animal models are used to predict how devices would react in human with vascular disease. Due to their size, accessibility, and damage response being comparable to human vessels, coronary arteries of porcine and iliac arteries of rabbits are acceptable for pre-clinical testing. It is crucial to choose device with right dimensions for animal model artery, since too much mechanical stent damage might compromise safety and effectiveness data. Regardless of the cause of mortality, all animals which experience death or other undesirable clinical outcomes should be checked, and the stent status should be thoroughly recorded. In all animal models, standard anti-platelet treatment should be used.
- Porcine Coronary Artery Model: The porcine model has normal cholesterol levels, the stents are implanted into coronary arteries that have not previously been injured with stent and artery ratio of 1.0: 1.1).
- Rabbit Iliac Artery Model: Rabbits can have normal or elevated cholesterol levels. Similar to the pig model, stents should be placed in healthy arteries which have not been previously injured with stent and artery ratio of 1.0: 1.1. The lesser variability in damage and inflammation following stent insertion is a benefit of the rabbit iliac artery model, which makes it useful for research into the biocompatibility and safety of experimental devices. The rabbit model may provide significant benefits over swine with regard to the time course of re-endothelialization, which is slower in rabbits than in swine [17-19] , particularly for research focusing on the re endothelialization of devices.
Standards for Evaluation are Necropsy Evaluation, Tissue Processing and Fixation, Histopathology, Clinical Observations and Blood Work, Overlapping Stents and Long Stents, Intravascular Imaging [20-23] , Statistical Comparison, Time Point of Follow up, In vitro and in vivo pharmacokinetics, Dose Finding and Biochemical analysis of degradation products.
VI. PRE-CLINICAL EVALUATION ASSESSMENT
The manufacturer's practices and records of pre-clinical evaluation must be assessed by the notified body to obtain CE mark. The company's processes and documentation relating to:
- Pre-clinical studies;
- Risk management;
- Analysis of data regarding compliance to required standards;
- Validation and verification data;
- Stability and packaging of final product;
- Labeling information and
- The conclusions drawn from pre-clinical evaluation shall be examined and verified by the notified body.
The NB must clearly explain its findings about the asserted equivalency as well as the applicability and sufficiency of the evidence to support compliance. Include a pre-clinical assessment report as a part of the examination report of the EU type mentioned and clearly state the results of its evaluation.
VII. CERTIFICATE OF CONFORMANCE OR CE MARKING IN EUROPE
Certificate of Conformance or CE mark is nothing but the Compliance Label. By using the CE mark as in Fig 2, a company assures whether a product complies to the appropriate regulations before it enter into the market. The primary goal is to prove that a device is secure and therefore it performs as intended by the manufacturer.

The Notified Body shall certify adherence to essential principles through Certificate of conformance in a way to enter into EU market. The Regulatory authority examines such statement, with the sole exception of Class I devices. MDs shall adhere to the Regulation (EU) 2017/746 and Regulation (EU) 2017/745 to get the CE mark and get marketed. However, drug-eluting stents have a few more special conditions that must be satisfied. The manufacturer must pay close attention to the following Directive requirements in order to apply the CE Marking:
- Classification;
- Clinical Evaluation;
- Essential Principles;
- Conformity Assessment;
- Labeling and Languages;
- Technical Documentation and
- Post-Marketing Surveillance.
The company needs to use an NB to get authorization for coronary stents. The responsibility of NBs is to study a technical dossier given by the manufacturer, test samples of the device if necessary, and analyze the offered data in regard to both nonclinical and clinical assessment. Following the issuance of a certificate, the product can bear the CE mark.
A. Validity of CE Marking Certificate
While CE Marking certifications normally have three-year validity, they are yearly verified in conjunction with the ISO 13485 monitoring audit. They continue to be effective as long as you don't alter the device design, its intended application and its usage functions. The CE certificate will be revoked if the manufacturer fails in surveillance audit. The list of CE mark approved stents is shown in Table 4.
|
S. No |
Stent Name |
Approved Year |
Company |
|
1 |
Biomime |
2011 |
Meril Life Sciences |
|
2 |
Cypher |
2012 |
Cordis Corporation |
|
3 |
Taxus |
2013 |
Boston Scientific |
|
4 |
Self Apposing Stent |
2013 |
Stentys Med Technologies |
|
5 |
Xience |
2013 |
Abbott |
|
6 |
Ultimaster |
2014 |
Terumo |
|
7 |
EluNIR |
2017 |
Bionics [24] |
|
8 |
Orsiro |
2020 |
Biotronik [25] |
|
9 |
Inspiron |
2020 |
Scitech |
|
10 |
Onyx Frontier™ |
2022 |
Medtronic [26] |
Table 4: CE marked approved stents.
VIII. MARKET APPROVAL PROCESS FOR CLASS III MEDICAL DEVICES IN EU
As Drug Eluting Stents are categorized in to Class III devices, the regulatory process for market approval is as following Fig. 3:
|
1. Decide which regulation applies either Regulation (EU) 2017/746 Medical Device or Regulation (EU) 2017/745- Active implantable medical device. |
|
2. These Medical Device Directives classify these Drug-Eluting stents as Class-III. |
|
3. Implement a quality management system (QMS) through ISO-13485 international standards to achieve QMS compliance. |
|
4. Create a Design Dossier, often known as a Technical File. |
|
5. If the manufacturers haven't a site in Europe, choose an Experienced Attorney there who is qualified to deal with regulatory matters. |
|
6. Upon accomplishment of a surveillance audit adequately, the concerned authority will provide an ISO 13485 certificate. |
|
7. Create a Declaration of Conformity (DoC) which certifies that the product complies to the EU regulations. |
|
8. The CE marking is now able to apply to the label by device maker. |
|
9. A few EU members demand further licensing for class IIa, IIb, or class III devices before they may be sold in local markets. |
|
10. NB will carry out a yearly audit to verify adherence to Regulation (EU) 2017/746 and Regulation (EU) 2017/745. A company's accreditation for the CE marking can no longer is valid if the inspection is unsuccessful. The firm must update CER and Post Market Surveillance tasks [27]. |
Fig. 3: Market approval process for medical devices in EU.
A. Design Dossier Examination
DES was especially susceptible to evaluation of the device's performance as they are Class III devices. An authorized body will receive a request from the maker for an evaluation of a product's design dossier. The product's concept, manufacturing methods, including functionality must all be detailed in the examination. A Summary technical document (STED) is needed from the manufacturer. The product's EU design-examination report is delivered to manufacturer by the regulatory authority after the inspection. A designated member shall grant an EU design-examination certificate containing the effective date and examination results.
IX. FAILURE MODES AND CLINICAL CONSEQUENCES OF DES
There are associated failure modes and potential clinical risks with each of the device component as listed in Table 5.
|
Device Components |
Failure Modes |
Potential Clinical Risks |
|
Stent platform |
Stent Fracture, lack of visibility, Stent recoil, longitudinal deformation, non-optimal radial stiffness, crimped profile and flexibility, biocompatibility |
Stent thrombosis, restenosis, deliverability, geographical Miss, need for additional stenting, inflammation, myocardial inflammation |
|
Stent coating |
Lack of coating integrity, uniformity, Biocompatibility |
Stent thrombosis, restenosis, myocardial infarction, edge effects, inflammation |
|
Medicinal substance |
Toxicity, biocompatibility |
Delayed healing, stent thrombosis, hypersensitivity, prolonged anti-platelet therapy |
|
Delivery system |
Flexibility, Pushability, particulate generation, shaft kinking, stent Securement, balloon rupture |
Procedural success, embolism, vascular injury, stent loss, thrombosis, vessel damage |
Table 5: Stent Failure Modes and Clinical Consequences.
Conclusion
The first endovascular treatment percutaneous angioplasty has negative remodeling which was overcome later by Drug eluting stents. Today drug eluting stents are most widely used in coronary diseases leading to significantly better patient outcomes after a percutaneous intervention. The therapeutic efficacy of coronary DES must be careful examined by the Notified body and Component authority in EU to determine the compliance of product to the regulatory standards. To market a medical device in European Union, a manufacturer must get CE certification by regulatory authority after determining the product compliance. The pre-clinical studies of DES must follow the standards of ISO -10993, 10555 and ISO- 25539. The product development, with a focus on Pre-clinical studies for DES must adhere to the device deficiency reporting requirements and Post market surveillance system as per the regulations.
References
[1] Nagod, Shilpa. (2019). A Review on Drug Eluting Stents in Treating Hepatic Artery Stenosis Post Liver Transplantation. International Journal for Research in Applied Science and Engineering Technology. 7. 1993-1996. 10.22214/ijraset.2019.6335. [2] Cherly Whitten; What To Known About Drug-Eluting Stents; James Beckerman, MD, FACC; on October 21, 2022; https://www.webmd.com/heart-disease/what-to-know-about-drug-eluting-stents. [3] Report Of The Esc-Eapci Task Force On The Evaluation Of Coronary Stents; European society of cardiology:https://www.escardio.org/static_file/Escardio/Subspecialty/EAPCI/Documents/ESC_EAPC I_TF_StentEvaluationDocument_Revision_final-1.pdf [4] Robert A. Byrne, Patrick W. Serruys, Andreas Baumbach, Javier Escaned, Jean Fajadet, Stefan James, Michael Joner, Semih Oktay, Peter Jüni, Adnan Kastrati, George Sianos, Giulio G. Stefanini, William Wijns, Stephan Windecker; Report of a European Society of Cardiology-European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: executive summary; European Heart Journal, Volume 36, Issue 38, 7 October 2015, https://doi.org/10.1093/eurheartj/ehv203 [5] European Commission; New regulations; https://health.ec.europa.eu/medical-devices-sector/new-regulations_en [6] Byrne, Robert & Serruys, Patrick & Baumbach, Andreas & Escaned, Javier & Fajadet, Jean & James, Stefan & Joner, Michael & Oktay, Semih & Juni, Peter & Kastrati, Adnan & Sianos, George & Stefanini, Giulio & Wijns, William & Windecker, Stephan. (2015). Report of a European Society of Cardiology-European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: Executive summary. European heart journal. 36. 10.1093/eurheartj/ehv203. [7] Bsigroups; Want to know more about notified body; https://www.bsigroup.com/meddev/LocalFiles/en-SG/Services/BSI-md-notifed-body-guide-brochure-UK-EN.pdf [8] Council; Council Directive 93/42/EEC; 14 june 1993; Official Journal of the European Communities: https://eurlex.europa.eu/legalcontent/EN/TXT/PDF/?uri=CELEX:31993L0042 [9] Paula McCarthy; How to CE Mark a Medical Device that Incorporates a Drug; August 19, 2015; MedTech Intelligence; https://www.medtechintelligence.com/feature_article/how-to-ce-mark-a-medical-device-that-incorporates-a-drug/ [10] Lee DH, de la Torre Hernandez JM. The Newest Generation of Drug-eluting Stents and Beyond. Eur Cardiol. 2018 Aug;13(1):54-59. doi: 10.15420/ecr.2018:8:2. PMID: 30310472; PMCID: PMC6159420. [11] ISO 25539-2:2020 Cardiovascular implants-Endovascular devices- Part 2: Vascular stents; Sept 2020: https://www.iso.org/standard/69835.html [12] ISO 10993-1:2018 Biological Evaluation of Medical Devices; Part-1 Evaluation and testing Risk management process; Aug 2018: https://www.iso.org/standard/68936.html [13] ISO 10555-1: 2013/ Amd 1:2017 Intravascular Catheters Sterile and Single use catheters- Part: 1- General requirements; Nov 2017: https://www.iso.org/standard/70319.html [14] European Medicines Agency; GUIDELINE ON THE DEVELOPMENT OF MEDICINAL SUBSTANCES CONTAINED IN DRUG-ELUTING (MEDICINAL SUBSTANCE-ELUTING) CORONARY STENTS; 22 March 2007; https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-development-medicinal-substances-contained-drug-eluting-medicinal-substance-eluting_en.pdf [15] Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008 Jul; 29(20):2941-53. doi: 10.1016/j.biomaterials.2008.04.023. Epub 2008 Apr 28. PMID: 18440630. [16] Mani G, Feldman MD, Patel D, Agrawal CM. Coronary stents: a materials perspective. Biomaterials. 2007 Mar;28(9):1689-710. doi: 10.1016/j.biomaterials.2006.11.042. Epub 2006 Dec 22. PMID: 17188349. [17] Schwartz RS, Edelman ER, Carter A, Chronos N, Rogers C, Robinson KA, Waksman R, Weinberger J, Wilensky RL, Jensen DN, Zuckerman BD, Virmani R; Consensus Committee. Drug-eluting stents in preclinical studies: recommended evaluation from a consensus group. Circulation. 2002 Oct 1;106(14):1867-73. doi: 10.1161/01.cir.0000033485.20594.6f. PMID: 12356643. [18] Faxon DP, Balelli LA, Sandborn T, Haudenschild C, Valeri R, Ryan TJ. The effect of antiplatelet therapy on platelet accumulation after experimental angioplasty in the rabbit iliac model. Int J Cardiol. 1992 Jul;36(1):41-7. doi: 10.1016/0167-5273(92)90106-d. PMID: 1428251. [19] Malle C, Tada T, Steigerwald K, Ughi GJ, Schuster T, Nakano M, Massberg S, Jehle J, Guagliumi G, Kastrati A, Virmani R, Byrne RA, Joner M. Tissue characterization after drug-eluting stent implantation using optical coherence tomography. Arterioscler Thromb Vasc Biol. 2013 Jun;33(6):1376-83. doi: 10.1161/ATVBAHA.113.301227. Epub 2013 Mar 28. PMID: 23539216. [20] Nissen SE, Gurley JC, Grines CL, Booth DC, McClure R, Berk M, Fischer C, DeMaria AN. Intravascular ultrasound assessment of lumen size and wall morphology in normal subjects and patients with coronary artery disease. Circulation. 1991 Sep;84(3):1087-99. doi: 10.1161/01.cir.84.3.1087. PMID: 1884441. [21] Mintz GS, Kent KM, Pichard AD, Satler LF, Popma JJ, Leon MB. Contribution of inadequate arterial remodeling to the development of focal coronary artery stenoses. An intravascular ultrasound study. Circulation. 1997 Apr 1;95(7):1791-8. doi: 10.1161/01.cir.95.7.1791. PMID: 9107165. [22] Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. Optical coherence tomography. Science. 1991 Nov 22;254(5035):1178-81. doi: 10.1126/science.1957169. PMID: 1957169; PMCID: PMC4638169. [23] Kawase Y, Hoshino K, Yoneyama R, McGregor J, Hajjar RJ, Jang IK, Hayase M. In vivo volumetric analysis of coronary stent using optical coherence tomography with a novel balloon occlusion-flushing catheter: a comparison with intravascular ultrasound. Ultrasound Med Biol. 2005 Oct;31(10):1343-9. doi: 10.1016/j.ultrasmedbio.2005.05.010. PMID: 16223637. [24] Michael O’Riordan, FDA Approves EluNIR Drug Eluting Stent, tctmd news, Nov 30, 2017. https://www.tctmd.com/news/fda-approves-elunir-drug-eluting-stent [25] Biotronik, Buelach, Biotronic introduces next generation Drug Eluting Stent System in the CE region; February 21, 2020. https://news.biotronik.com/biotronik-introduces-next-generation-drug-eluting-stent-system-in-the-ce-region/ [26] Medtronic. Medtronic launches latest generation drug-eluting coronary stent system following CE Mark approval. Aug 24, 2022. Available from: https://libguides.murdoch.edu.au/Vancouver/newspaper. [27] World Health Organization; Medical device regulations: Global overview and guiding principles;2003; https://apps.who.int/iris/handle/10665/42744
Copyright
Copyright © 2023 Shailaja P., Snehalatha. G, Revathi. K, Likhita. P. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET50658
Publish Date : 2023-04-19
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

