Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Preparation and Characterization of CuFeS2 Nanoparticles Synthesized via Hydrothermal Method
Authors: S Vigneswaran, P. Gowthaman, S. Sangeethavanathi, M. Sathishkumar
DOI Link: https://doi.org/10.22214/ijraset.2024.62599
Certificate: View Certificate
Abstract
This research involved the successful synthesis of CuFeS2 nanoparticles using a hydrothermal method. The CuFeS2 nanoparticles were characterized using various analytical techniques, including X-ray diffraction (XRD), Raman spectroscopy, field emission scanning electron microscopy (FESEM), energy-dispersive X-ray spectroscopy (EDX), UV-Visible spectroscopy, and Brunauer-Emmett-Teller (BET) surface area analysis. The tetragonal crystalline structure was determined using X-ray diffraction (XRD) examination, indicating a particle size of 14 nm. The existence of chalcopyrite CuFeS2 nanoparticles was established by the analysis of Raman spectra. The amorphous morphology was observed by FESEM imaging. The existence of Cu, Fe, and S elements was verified using EDX analysis, with no significant impurities observed. The UV-Visible investigation revealed a significant capacity for absorption within the wavelength region of 500-600 nm, accompanied by an energy band gap of 2.35 eV. The examination of BET surface area revealed a surface area of 62 m²/g and a pore size of 10 nm. The obtained findings suggest that the CuFeS2 nanoparticles possess favorable properties that render them appropriate for use in photocatalytic applications.
Introduction
I. INTRODUCTION
Nanomaterials have attracted considerable interest in recent years owing to their distinctive characteristics and possible uses in industries including electronics, catalysis, sensing, and environmental remediation [1-2]. Transition metal chalcogenides have garnered significant attention as potential nanomaterials due to their unique electrical, optical, and catalytic characteristics [3-5]. Chalcopyrite copper iron sulfide (CuFeS2) is a semiconductor compound that belongs to the I-III-VI2 ternary group. The material has distinctive characteristics, including a high Neel temperature and outstanding electrical and optical capabilities, characterized by a very narrow optical band gap [6]. CuFeS2 sometimes referred to as chalcopyrite, is a ternary compound that has intriguing properties such as a small bandgap, high absorption coefficient, and exceptional chemical stability. CuFeS2 nanoparticles possess these characteristics that render them very appealing for use in solar cells, photocatalysis, and photovoltaic systems [7].
Nevertheless, the achievement of successful synthesis and comprehensive characterization of CuFeS2 nanoparticles is crucial in order to fully harness their capabilities in many technological domains. Within this particular context, the hydrothermal approach emerges as a very adaptable and efficient methodology for the production of nanoparticles, exhibiting meticulous control over their dimensions, structure, and crystalline properties [8]. The characteristics of the resultant nanoparticles may be customized to match particular needs by manipulating reaction parameters, including temperature, pressure, and precursor concentrations. Several synthesis methods have been explored to fabricate CuFeS2 nanoparticles, each offering distinct advantages and challenges. The synthesis method involves the controlled hydrothermal reaction of copper, iron, and sulfur precursors to generate nanoparticles that are uniform and well-defined. Among these methods, the hydrothermal synthesis route has gained significant attention for its ability to produce nanoparticles with controlled size, morphology, and crystallinity. The hydrothermal method involves the reaction of precursor materials under elevated temperature and pressure conditions in an aqueous solution, facilitating the nucleation and growth of CuFeS2 nanoparticles with tailored properties [9-10].
Understanding the structural and optical properties of CuFeS2 nanoparticles is crucial for optimizing their synthesis and harnessing their potential in various applications. The crystal structure of CuFeS2, characterized by a tetragonal lattice arrangement, influences its electronic and optical behaviors [11].
Additionally, the bandgap of CuFeS2 can be tuned by adjusting its composition and morphology, enabling tailored absorption properties for specific applications. The optical properties of CuFeS2, including its absorption and emission spectra, play a pivotal role in applications such as photovoltaics, photocatalysis, and sensing. CuFeS2 nanoparticles exhibit unique optical properties arising from quantum confinement effects and surface plasmon resonance phenomena, which can be exploited for efficient light harvesting and charge separation in photovoltaic devices, as well as for enhancing catalytic activity in photocatalytic reactions [12]. Furthermore, CuFeS2 nanoparticles have garnered attention for their promising applications in various fields, including solar cells, photoelectrochemical devices, gas sensors, and biomedical imaging. The tunable bandgap and high absorption coefficients of CuFeS2 make it an ideal candidate for photovoltaic applications, while its catalytic activity and biocompatibility render it suitable for sensing and biomedical applications [13].
In this study, we embark on a comprehensive exploration of CuFeS2 nanoparticles fabricated through the hydrothermal method, delving into their production, analysis, and prospective applications. Through meticulous synthesis processes, we successfully generate CuFeS2 nanoparticles, followed by an exhaustive evaluation employing a diverse array of analytical techniques. Our findings underscore the remarkable potential of these nanoparticles across various technological domains, with particular emphasis on their applicability in photocatalysis.
II. EXPERIMENTAL SECTION
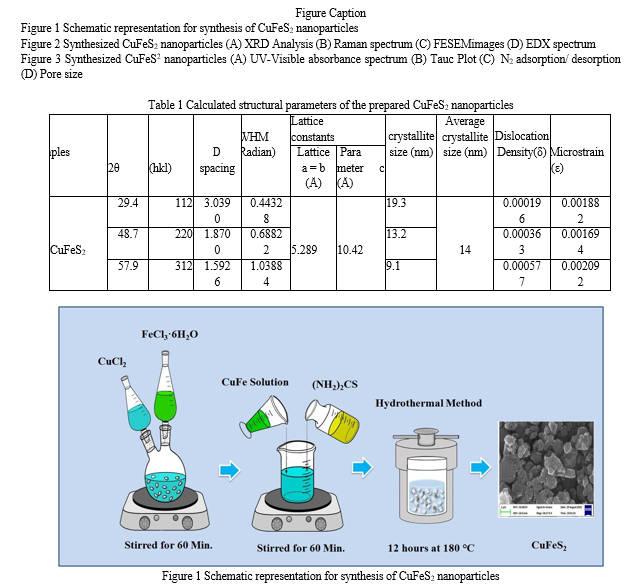
The hydrothermal method was employed for the facile synthesis of CuFeS2 particles. Initially, copper chloride (1 mol; CuCl2; Sigma–Aldrich) and ferric chloride (1 mol; FeCl36H2O; Sigma– Aldrich) were dissolved in 100 mL of pure water under continuous stirring for duration of 60 minutes. Following this, thiourea (1 mol, (NH2)2CS, Sigma–Aldrich) was gradually added to the CuFe solution, reaching a volume of 100 ml. The resulting CuFeS2 solution was then transferred into a Teflon-lined stainless steel autoclave and maintained at a temperature of 180°C for a period of 12 hours. After the hydrothermal reaction, the obtained powders underwent filtration and were thoroughly washed with hot distilled water before undergoing vacuum drying. The residual substance was collected, washed with deionized water, and subsequently incubated overnight at 45°C to ensure complete drying. Additionally, a schematic representation of the experimental setup is provided in Figure 1.
The synthesized CuFeS2 nanoparticles underwent comprehensive characterization utilizing state- of-the-art analytical techniques to elucidate their structural, morphological, elemental composition, surface area, and optical properties. Structural analysis was performed using a high-resolution X-ray diffractometer (XRD) (XPERT-PRO) to determine the crystalline structure, while Raman spectroscopy (WITech CRM200) provided insights into the molecular vibrational modes. Morphological and elemental composition analysis was carried out using a Field Emission Scanning Electron Microscope (FESEM) (Sigma HV – Carl ZEISS) equipped with Bruker Quantax 200-Z10 Energy-Dispersive X-ray Spectroscopy (EDS) detector. The surface area of the nanoparticles was determined using a Nova 2200e Analyzer through N2 adsorption and desorption processes. Optical properties were investigated using UV-Vis spectrometer (Hitachi-UH5300, λ = 200–900 nm) to assess their absorbance characteristics in the ultraviolet-visible range. These characterization techniques collectively provided a comprehensive understanding of the synthesized CuFeS2 nanoparticles, paving the way for their potential applications in various technological fields.
III. RESULTS AND DISCUSSION
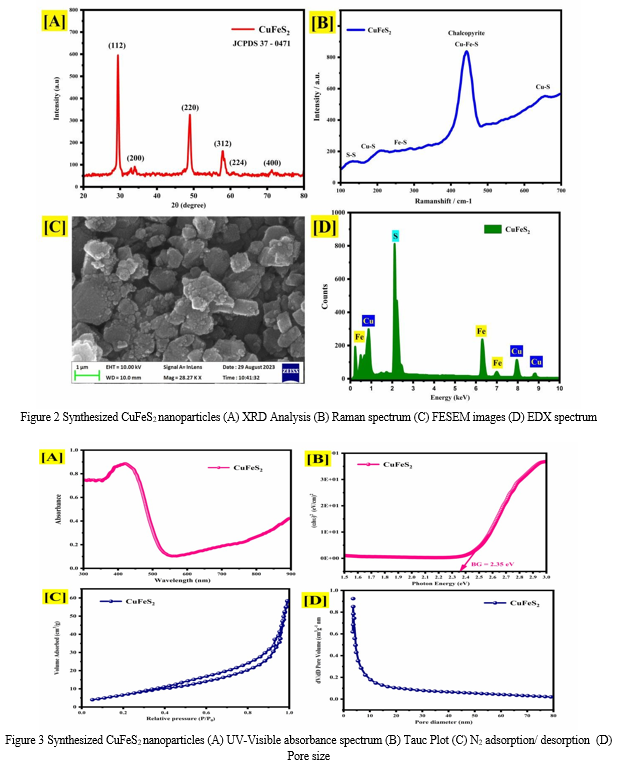
Figure 2(A) presents the X-ray diffraction (XRD) analysis results of the synthesized CuFeS2 nanoparticles. The diffraction pattern exhibits prominent peaks located at 2θ angles of 29.4º, 48.7º, and 59.7º, which correspond to the (112), (200), and (312) lattice planes, respectively. These peaks confirm the crystalline nature of the synthesized CuFeS2 nanoparticles, with the observed pattern closely matching the characteristic peaks of tetragonal chalcopyrite CuFeS2 as documented in the Joint Committee on Powder Diffraction Standards (JCPDS) card no. 37-0471. Notably, the peak at 29.4º corresponding to the (112) lattice plane exhibits the highest intensity, indicating its predominant presence in the synthesized CuFeS2 nanoparticles. The Scherrer formula [14] was applied to estimate the crystallite size, revealing an average particle size of approximately 14 nm. This result underscores the influence of the hydrothermal method in enhancing the crystalline structure and controlling the particle size of CuFeS2 nanoparticles. Based on the structural parameters obtained from XRD analysis (Table 1), the synthesized CuFeS2 nanoparticles exhibit distinct features indicative of their crystalline nature and internal strain. The full width at half maximum (FWHM) values corresponding to the (112), (220), and
(312) lattice planes are 0.44328, 0.68822, and 1.03884 radians, respectively. These FWHM values reflect the degree of peak broadening, providing insights into the CuFeS2 crystallite size distribution and internal strain within the nanoparticles.
Furthermore, the dislocation density (δ) and microstrain (ε) parameters offer valuable information about the structural defects and strain- induced distortions present in the synthesized CuFeS2 nanoparticles. The observed microstrain values provide insights into the extent of lattice distortion and internal stress within the CuFeS2 nanoparticles. Overall, the structural parameters obtained from XRD analysis elucidate the crystalline characteristics, internal strain, and defect density of the synthesized CuFeS2 nanoparticles, contributing to a comprehensive understanding of their structural properties and potential applications.
The Raman spectrum of the synthesized CuFeS2 sample is depicted in Figure 2(B), revealing several distinct peaks within the 100-700 cm-1 range. Specifically, peaks are observed at wavenumbers of 151, 184, 323, 449, and 659 cm-1. The presence of Cu-S bonds is indicated by peaks observed at 184 and 659 cm-1, while the presence of Fe-S bonds is evidenced by the peak at 323 cm-1. Additionally, a prominent peak corresponding to sulfur is observed at 183 cm-1. The peak at 151 cm-1 suggests the presence of S-S bonds. The dominant signal at 659 cm-1 further confirms the presence of CuFeS2 on the mineral surface of chalcopyrite. Chalcopyrite exhibits a complex stratified arrangement, with individual sulfur atoms within layers and sulfur dimers covalently bonded between layers. The observed Raman peaks provide insights into the molecular structure and bonding configuration of CuFeS2, highlighting its unique characteristics and potential application [15-16].
Figure 2(C) presents the field emission scanning electron microscopy (FESEM) image of the synthesized CuFeS2 sample. The FESEM imaging revealed an amorphous morphology, indicating the absence of well-defined crystalline structures. One possible reason for observing an amorphous morphology in synthesized CuFeS2 nanoparticles via the hydrothermal method could be the rapid nucleation and growth kinetics under the specific reaction conditions employed. In the hydrothermal synthesis process, precursor materials are reacted in an aqueous solution under elevated temperature and pressure conditions, promoting the formation of nanoparticles through nucleation and subsequent growth [17]. Additionally, energy-dispersive X-ray spectroscopy (EDX) analysis was conducted to verify the elemental composition of the synthesized CuFeS2 nanoparticles. Figure 2(D) shows the EDX spectrum, confirming the presence of copper (Cu), iron (Fe), and sulfur (S) elements in the sample. Importantly, no significant impurities were detected in the EDX analysis, underscoring the purity of the synthesized CuFeS2 nanoparticles.
The UV-Visible absorbance spectra of the synthesized CuFeS2, as depicted in Figure 3(A) within the wavelength range of 300-900 nm, revealed a distinct peak absorption observed prominently near 550 nm. This absorption behavior indicates the CuFeS2 interaction with electromagnetic radiation in the visible region, suggestive of its potential optical applications. Further analysis involved the calculation of the CuFeS2 band gap, achieved through the Tauc plot method illustrated in Figure 3(B), resulting in a determined value of 2.35 eV. The enhanced band gap observed in CuFeS2 synthesized via the hydrothermal method can be attributed to several factors. The controlled growth conditions inherent to the hydrothermal synthesis process enable precise manipulation of reaction parameters, leading to the formation of materials with tailored electronic properties [18]. Additionally, this method facilitates the minimization of defects within the crystal lattice, ensures uniform particle size and distribution, and allows for better control over stoichiometry. Collectively, these factors contribute to the modification of the CuFeS2 electronic band structure, ultimately resulting in the observed increase in the band gap, thus highlighting the efficacy of the hydrothermal method in tailoring the optical properties of CuFeS2 for various applications
The surface area analysis of the synthesized CuFeS2 nanoparticles, as depicted in Figure 3(C), involved examining the adsorption-desorption isotherms of nitrogen molecules to characterize the material's surface area and pore structure. The analysis revealed a significant surface area of 62 m²/g and a corresponding pore size of 10 nm, as illustrated in Figure 3(D). Notably, the nitrogen adsorption-desorption isotherm displayed type III behavior with hysteresis loops, indicating the presence of mesoporous characteristics within the CuFeS2 structure. The improved surface area observed for CuFeS2 synthesized via the hydrothermal method can be attributed to several factors inherent to this synthesis technique [19]. Hydrothermal synthesis provides a controlled environment conducive to the formation of well-defined crystalline structures with high surface area-to-volume ratios. Additionally, the precise control over reaction parameters such as temperature, pressure, and pH facilitates the creation of nanoparticles with uniform size and distribution, thereby enhancing the overall surface area. Furthermore, the mesoporous characteristics observed in the synthesized CuFeS2 nanoparticles suggest the presence of interconnected pores, which can contribute to the increased surface area. Overall, the hydrothermal method enables the synthesis of CuFeS2 nanoparticles with improved surface area, making them promising candidates for various applications. Moreover, hydrothermal synthesis allows for precise control over reaction parameters, leading to the formation of nanoparticles with uniform size and distribution. Furthermore, the hydrothermal environment promotes the growth of crystalline structures with high surface area-to-volume ratios. These factors collectively contribute to the enhanced surface area of CuFeS2 nanoparticles synthesized via the hydrothermal method, rendering them promising candidates for various applications, including catalysis, sensing, and energy [20].
Conclusion
In conclusion, the hydrothermally synthesized CuFeS2 nanoparticles were characterized using XRD, Raman, FESEM, EDX, UV-Visible, and BET surface area analysis. XRD revealed the CuFeS2 nanoparticles exhibits the tetragonal chalcopyrite CuFeS2 structure and the average particle size was 14 nm, indicating that the hydrothermal process controls particle size. FESEM imaging showed an amorphous morphology of CuFeS2, probably owing to fast nucleation and growth kinetics. Raman spectroscopy revealed its molecular structure and bonding arrangement. EDX examination showed CuFeS2 nanoparticles elemental composition without contaminants. UV-Visible spectroscopy showed strong absorption and a band gap of 2.35 eV, increased by controlled growth conditions and hydrothermal defect reduction. Additionally, BET surface area study showed a mesoporous surface area of 62 m²/g due to regulated synthesis and linked pore development. These results show that the hydrothermal approach can tune CuFeS2 nanoparticles structural and optical characteristics, making them suitable for catalysis, sensing, and energy conversion.
References
[1] Dan Niu, Yijun Liu, Qunhu Xue, Zhihong Wu, Cheng Yao, Xinyu Guo, Anwen Ren, Peng Li, Fabricating FeS2 and CuFeS2 microspheres embedded into biomass derived porous carbon with excellent microwave absorption, Materials Today Communications, Volume 38, 2024, 108100, [2] Julia da Silveira Salla, Guilherme Luiz Dotto, Dachamir Hotza, Richard Landers, Katia da Boit Martinello, Edson Luiz Foletto, Enhanced catalytic performance of CuFeS2 chalcogenide prepared by microwave-assisted route for photo-Fenton oxidation of emerging pollutant in water, Journal of Environmental Chemical Engineering, Volume 8, Issue 5, 2020, 104077. [3] Nenguba Poloko, Gwiranai Danha, Tshepho Gaogane, Processing and characterization of chalcopyrite (CuFeS2) sample from Botswana, Procedia Manufacturing, Volume 35, 2019, Pages 488-493. [4] Bhoomi S. Shah, Jolly B. Raval, Deepak Kumar, Sunil H. Chaki, M.P. Deshpande, A review on ternary CuFeS2 compound: Fabrication strategies and applications, Journal of Alloys and Compounds, Volume 938, 2023, 168566. [5] Yang-Wei Lin, Ting-Yu Lai, Yu-Shu Pan, Xuan-Wei Fang, Hsing-Yi Chen, Chen-Hao Yeh, Tsunghsueh Wu, Enhanced catalytic performance of CuFeS2 chalcogenides for activation of persulfate towards decolorization and disinfection of pollutant in water, Materials Chemistry and Physics, Volume 301, 2023, 127564. [6] Yasmin Vieira, Gabriel Severo de Carvalho, Jandira Leichtweis, Clóvia Marozzin Mistura, Edson Luiz Foletto, Asad Nawaz, Salim Manoharadas, Renato Zanella, Guilherme Luiz Dotto, CuFeS2 /activated carbon heterostructure as a microwave- responsive catalyst for reductive and oxidative degradation of ibuprofen, ketoprofen, and diclofenac, Chemical Engineering Journal, Volume 480, 2024, 148060. [7] Sivakumar Bose, Sivaprakasam Radhakrishnan, Byoung-Suhk Kim, Hyun Wook Kang, Formulation of amorphous carbon embedded CuFeS2 hybrids for the electrochemical detection of Quercetin, Materials Today Chemistry, Volume 26, 2022, 101228. [8] Aleem Ansari, Rashmi A. Badhe, Dipak G. Babar, Shivram S. Garje, One pot solvothermal synthesis of bimetallic copper iron sulfide (CuFeS2) and its use as electrode material in supercapacitor applications, Applied Surface Science Advances, Volume 9, 2022, 100231. [9] Ubaid ur Rehman, Khalid Mahmood, Arslan Ashfaq, Adnan Ali, Sofia Tahir, Salma Ikram, Abdul Rehman, Kashaf ul Sahar, Waqas Ahmad, Nasir Amin, Enhanced thermoelectric performance of hydrothermally synthesized CuFeS2 nanostructures by controlling the Cu/Fe ratio, Materials Chemistry and Physics, Volume 279, 2022, 125765. [10] Changsheng Xu, Jie Wang, Kewei Wu, Guocui Xi, Xuebu Hu, Lei Qiu, CuFeS2 anchored in ethylenediamine-modified reduced graphene oxide as an anode material for sodium ion batteries, Materials Letters, Volume 308, Part B, 2022, 131164. [11] Junyi Huang, Yuanhao Zhou, Shimao Deng, Yangzi Shangguan, Ranhao Wang, Qiuyue Ge, Xuezhen Feng, Zhigang Yang, Yongfei Ji, Ting Fan, Baiyang Chen, Boqiang Li, Chunmiao Zheng, Xijun Hu, Hong Chen, Photo-assisted reductive cleavage and catalytic hydrolysis-mediated persulfate activation by mixed redox-couple-involved CuFeS2 for efficient trichloroethylene oxidation in groundwater, Water Research, Volume 222, 2022, 118885. [12] P. Rupa Ranjani, P.M. Anjana, R.B. Rakhi, Solvothermal synthesis of CuFeS2 nanoflakes as a promising electrode material for supercapacitors, Journal of Energy Storage, Volume 33, 2021, 102063. [13] M. Sathishkumar, M. Saroja, M. Venkatachalam, Influence of (Cu, Al) doping concentration on the structural, optical and antimicrobial activity of ZnS thin films prepared by Sol-Gel dip coating techniques, Optik, Volume 182, 2019, Pages 774-785. [14] Yunhui Wang, Xue Li, Yiyong Zhang, Xinyi He, Jinbao Zhao, Ether based electrolyte improves the performance of CuFeS2 spike-like nanorods as a novel anode for lithium storage, Electrochimica Acta, Volume 158, 2015, Pages 368-373. [15] Erika Dutková, Zdenka Buj?áková, Jaroslav Ková?, Ivan Škorvánek, María Jesus Sayagués, Anna Zorkovská, Jaroslav Ková?, Peter Baláž, Mechanochemical synthesis, structural, magnetic, optical and electrooptical properties of CuFeS2 nanoparticles, Advanced Powder Technology, Volume 29, Issue 8, 2018, Pages 1820-1826. [16] M.X. Wang, L.S. Wang, G.H. Yue, X. Wang, P.X. Yan, D.L. Peng, Single crystal of CuFeS2 nanowires synthesized through solventothermal process, Materials Chemistry and Physics, Volume 115, Issue 1, 2009, Pages 147-150. [17] K.M. Deen, E. Asselin, On the use of a naturally-sourced CuFeS2 mineral concentrate for energy storage, Electrochimica Acta, Volume 297, 2019, Pages 1079-1093. [18] C Boekema, A.M Krupski, M Varasteh, K Parvin, F van Til, F van der Woude, G.A Sawatzky, Cu and Fe valence states in CuFeS2, Journal of Magnetism and Magnetic Materials, Volumes 272–276, Part 1, 2004, Pages 559-561. [19] S.K. Pradhan, B. Ghosh, L.K. Samanta, Mechanosynthesis of nanocrystalline CuFeS2 chalcopyrite, Physica E: Low-dimensional Systems and Nanostructures, Volume 33, Issue 1, 2006, Pages 144-146. [20] Yu-Hsiang A. Wang, Ningzhong Bao, Arunava Gupta, Shape-controlled synthesis of semiconducting CuFeS2 nanocrystals, Solid State Sciences, Volume 12, Issue 3, 2010, Pages 387-390, [21] M. Sathishkumar, S. Geethalakshmi, Enhanced photocatalytic and antibacterial activity of Cu:SnO2 nanoparticles synthesized by microwave assisted method, Mater. Today: Proc. 20 (1) (2020) 54-63. [22] S. Kannan, N.P. Subiramaniyam, M. Sathishkumar, Investigation on the structural, optical and photocatalytic degradation properties of Zns/Mn:Zns thin films under visible light irradiation, Mater. Today: Proc. 38 (2) (2021) 907-912. [23] S. Kannan, N.P. Subiramaniyam, M. Sathishkumar, Effect of annealing temperature and Mn doping on the structural and optical properties of ZnS thin films for enhanced photocatalytic degradation under visible light irradiation, Inorg. Chem. Commun. 119 (2020) 108068. [24] M. Sathishkumar, M. Saroja, M. Venkatachalam, P. Gowthaman, S. Kannan, A. Balamurugan, rGO encapsulated ZnSphotocatalysts for enhanced hydrogen evolution, Mater. Lett. 323 (2022) 132534. [25] S.K. Mani, M. Saroja, M. Venkatachalam, T. Rajamanickam, Antimicrobial activity and photocatalytic degradation properties of zinc sulfide nanoparticles synthesized by using plant extracts, J. Nanostruct. 8 (2) (2018) 107-118. [26] S. Kannan, NP. Subiramaniyam, M Sathishkumar, A novel green synthesis approach for improved photocatalytic activity and antibacterial properties of zinc sulfide nanoparticles using plant extract of Acalyphaindica and Tridaxprocumbens, J. Mater. Sci.: Mater. Electron. 31 (12) (2020) 9846-9859. [27] M Sathishkumar, AT Rajamanickam, M Saroja, Characterization, antimicrobial activity and photocatalytic degradation properties of pure and biosynthesized zinc sulfide nanoparticles using plant extracts, J. Mater. Sci.: Mater. Electron. 29 (16) (2018) 14200- 14209.
Copyright
Copyright © 2024 S Vigneswaran, P. Gowthaman, S. Sangeethavanathi, M. Sathishkumar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET62599
Publish Date : 2024-05-23
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

