Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Preparation and Evaluation of Medicated Oral Jelly
Authors: Prof. Surywanshi. A. D , Hudge. S. J, Devshatwar. R. S, Chavan. S. I, Kotsulwar. S. S, Salunke. K. J
DOI Link: https://doi.org/10.22214/ijraset.2024.64072
Certificate: View Certificate
Abstract
Developed in the 20th century, oral medicinal jellies are still produced commercially and are still well-liked by customers. These are chewable solid dose forms that dissolve in the mouth or throat to provide either localized or systemic effects. They are taken orally. As a pharmacological composition, oral medicated jelly has some benefits but also certain drawbacks. They can be applied to the buccal, labial, gingival, and sublingual channels of drug administration, among other routes. These jellies, which come in flavors including chocolate, strawberry, pineapple, and mango, are over-the-counter drugs. They comprise medications for a number of ailments, including sore throat, antistatic, erectile dysfunction, arthritis, and hypertension. The Japanese Ministry of Health, Labour, and Welfare authorized a once-weekly oral program in 2012 The world\'s first medication for osteoporosis in a jelly formulation was approved by the Japanese Ministry of Health, Labour and Welfare and is to be taken orally once a week. A process of heating and congealing is used in their formulation. Oral medicated jellies serve as a new dosage form with vast uses in pharmaceuticals, nutraceuticals, and over-the-counter drugs. This study offers formulation scientists insight into new uses for this drug delivery technology by focusing on several facets of oral medicated jelly formulation. Orodispersible dose formulations provide patients additional possibilities for medication administration. They are simple to use and don\'t need ingesting big, solid dose forms or consuming a lot of water. This research aims to develop and assess the mouth-dissolving film of lisinopril as an ACE inhibitor for the treatment of congestive heart failure and hypertension. When compared to traditional solid oral dose forms, these forms are thought to increase the bioavailability of medications.
Introduction
I. INTRODUCTION
Owing to its many benefits over alternative methods, the oral route is the one that is most frequently employed for medication administration. To address some limitations pertaining to particular patient populations, such as elderly and pediatric patients who experience difficulties ingesting or digesting solid dose forms, more developments are still necessary.
In order to provide pediatric and elderly patients who have trouble swallowing conventional oral solid dose forms with an alternative to tablets, capsules, and syrups, fast-dissolving drug delivery systems were created in the late 1970s. A thin strip is applied to the patient's tongue or any other oral mucosal tissue in these systems. For oromucosal and intragastric absorption, the film attaches and hydrates quickly before dissolving and releasing the medication. For the treatment of hypertension, mouth-dissolving films containing lisinopril provide an alternative to traditional tablets, syrups, and suppositories. Angiotensinconverting enzyme inhibitors such as lisinopril are used to treat excessive blood pressure, congestive heart failure (CHF), and to increase the likelihood of survival following a heart attack. The medication has a modest dose of 10 mg, and when taken once daily, it is proven to have a 24-hour duration of action. It is discovered that the drug is absorbed from the GI tract (oral) slowly and incompletely, reaching peak plasma levels after 7 hours. The objective of this research is to create and examine a film that dissolves in the mouth without the need for water. This facilitates simple swallowing, raises the drug's bioavailability, and enables a prompt commencement of action. In this study, hydroxypropyl methylcellulose (HPMC E15) and polyvinyl alcohol (PVA) in various ratios, along with aspartame for sweetening, citric acid for saliva stimulation, and glycerine for plasticizing, were used in an attempt to create a mouth-dissolving film of lisinopril. Oral medicated jellies are a type of edible solid medication that is taken orally and dissolves in the mouth or throat to provide either localized or systemic effects. They are well-liked since they often have minimal treatment costs and are non-invasive. Patient compliance is improved by oral drug delivery systems' safety, effectiveness, and affordability. Present pediatric formulations, however, have a number of shortcomings. Tablets, capsules, syrups, solutions, and drops comprise the majority of the paediatric formulations that are now on the market. A key factor in liquid formulations is dosage volume. For children under five years old, only dose volumes less than five milliliters are advised, and for children five years old and up, only dose volumes less than tenmilliliters.
Natural gums like tragacanth, pectin, and sodium alginate, as well as synthetic derivatives like methylcellulose and sodium carboxyl methyl cellulose, can be used to make oral medicinal jellies. They are chosen over oral liquids or tablets because of their appealing look, tasty flavor, and ease of use. Since it significantly affects patient compliance, the need for the creation of oral medicated jelly (OMJs) has grown over the past ten years. A large portion of the public values OMJs, especially those who have swallowing difficulties. All age groups are susceptible to dysphagia, or trouble swallowing, but it is more common in the geriatric and pediatric populations, as well as in institutionalized patients and those with difficulties from motion sickness, nausea, and vomiting.
Over time, the use of medicated jelly as a medicine delivery method has grown in popularity. Medicated jellies currently contain a number of components, such as medications with fast-acting beginning of action and medications with significant absorption sites in the small intestine and stomach. They can be made from synthetic derivatives of natural materials like sodium carboxyl methyl cellulose and methylcellulose, or from natural gums like tragacanth, pectin, and sodium alginate. When it comes to taking medications, kids can think that jelly is a better option than oral liquid or tablets. In addition to treating systemic illnesses, medicated jelly can be used locally to treat diseases of the oral cavity.
II. MATERIALS
It appears that Wilcure Remedies Pvt, Ltd. Indore (M.P.) gave you a gift sample of Glibenclamide. You also have analytical-grade guar gum, pectin, mannitol, citric acid, sodium citrate, methylparaben, and stevia. How can I use these materials to help you even more? Are you going to create a particular medication formulation? In order for me to provide the most pertinent information, kindly supply more specifics.
III. PREPARATION AND METHODS
The preparation of Montelukast Sodium oral jelly involves the following steps:
- Use various polymers in varied amounts to prepare the jellies.
- Get the sugar syrup ready.
- Slowly mix in the gelling agent while heating the sugar syrup.
- After the gelling agent has fully dissolved, stir in the stabilizers and solubilizers and bring to a boil, stirring constantly, for a few minutes.
- Stir the liquid continuously while adding the preservatives once it has completely dissolved.
- After that, slowly add the medication while stirring constantly. Next, add flavor and color.
- Make sure all of the jellies are well combined.
- Use filtered water to adjust the final weight.
- To produce jelly, pour the fluid into molds and allow it to cool to room temperature.
This method allows for the creation of a palatable oral jelly formulation of Montelukast Sodium, which can be beneficial for patients who have difficulty swallowing traditional solid-dosage forms.
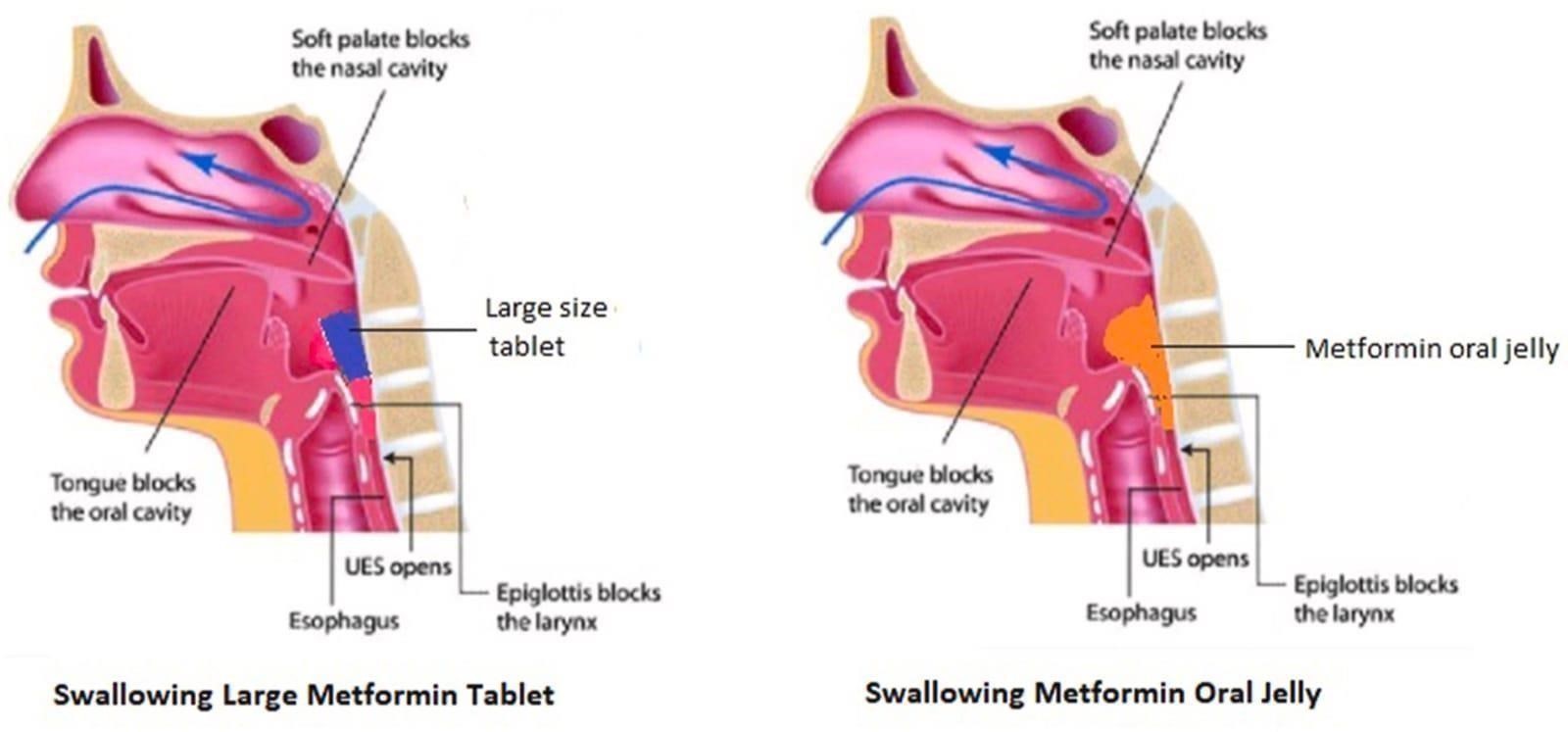 Fig.No.1 Difference Between Large Metformin Tablet And Metformin Oral Jelly Evaluation
Fig.No.1 Difference Between Large Metformin Tablet And Metformin Oral Jelly Evaluation
IV. PARAMETERS
A. pH
Jelly's stability and flavor are affected by its pH, it's true. Based on your measurements, the jelly has a pH of 8.15, which suggests a slight alkaline state. This could change the flavor, making it more bitter or soapy instead of sour. Since pH can have an impact on both the gelling process and the interaction between the ingredients, the stability of the jelly may also be impacted. The precise effect, however, might vary depending on the jelly's particular formula and ingredients. The way chemistry affects our meals never ceases to amaze me!
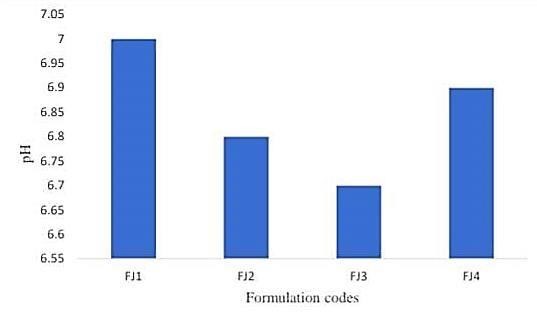
Fig.No.2 pH Determination Between Formulation Codes And pH
B. Viscosity
Using a Brookfield viscometer to measure the viscosity of jelly is a wonderful approach for testing the viscosity of a non-Newtonian fluid. It is true that spindle number 63 is appropriate for this type of examination. You should get accurate findings from the viscosity measurement at room temperature (25 °C ± 5 °C) at 50 rpm for a set duration of two minutes. Recall that viscosity is a crucial metric to manage in the food preparation process since it can impact the mouthfeel and texture of the jelly.
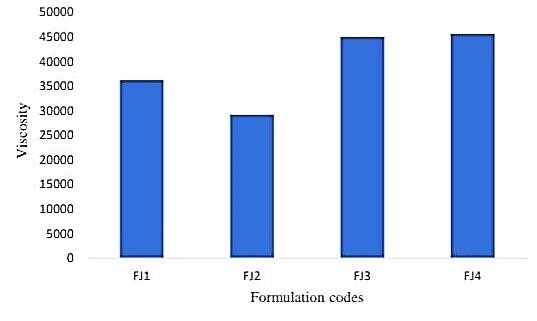
Fig.No.3 Viscosity Determination Between Formulation Codes And Viscosity
C. Spreadability
It appears that you are figuring out your jelly formulation's spreadability by following Multimer's advice. This technique, which uses weights, glass slides, and wooden blocks, is frequently used to gauge how spreadable semi-solid foods like jelly are. In this case, spreadability is typically calculated using the formula S = M × L/T, where S is spreadability, M is the weight attached to the top slide, L is the length of the glass slide (7.5 cm), and T is the time required to separate the two slides. Spreadability has a significant impact on consumer desire and is a crucial component of food texture. It's wonderful to see testing done with such thoroughness! Continue your fantastic effort!
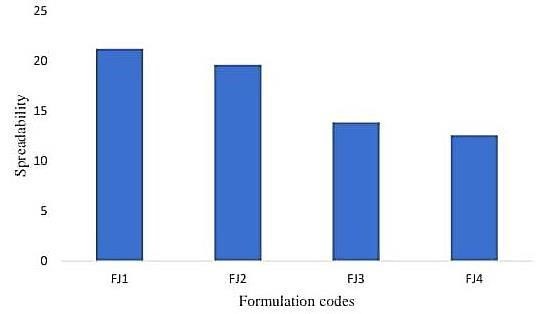
Fig.No.4 Spreadability Determination
D. Stickiness and Grittiness
After gently rubbing the jelly sample between two fingers, the product is visually inspected to determine the stickiness and grit level of the medicated jelly. The assessment is carried out to ascertain the jelly's stickiness and grittiness. Grittiness and stickiness are crucial characteristics to consider while assessing the jelly's texture. It is unclear, meanwhile, what the evaluation's scale is or how the findings would be applied.
|
Test |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Stickiness |
Non-Sticky |
Non-Sticky |
Non-Sticky |
Slightly sticky |
Slightly sticky |
Sticky |
|
Grittiness |
Less gritty |
Less gritty |
Less gritty |
Slightly gritty |
Slightly gritty |
More gritty |
|
Test |
F7 |
F8 |
F9 |
F10 |
F11 |
F12 |
|
Stickiness |
Slightly sticky |
Sticky |
Sticky |
Non-Sticky |
Slightly sticky |
Sticky |
|
Grittiness |
Slightly gritty |
More gritty |
More gritty |
Less gritty |
Slightly gritty |
More gritty |
Table. No. 01 Stickiness And Grittiness Of Formulated Jellies
While batches F4, F5, F7, F11, and F6, F8, F9, and F12 are sticky, batches F1, F2, F3, and F10's medicated jelly is not sticky. F6, F8, F9, and F12 exhibit greater grit, whilst F1, F2, F3, and F10 exhibit less grit and F4, F5, F7, and F11 exhibit somewhat grit.
V. CONTENT UNIFORMITY
A quality control test called the content uniformity test makes sure that each dosage form has the same amount of drug substances—that is, the batch's active pharmaceutical components. To create a 100 ug/ml solution, one medicated jelly from each formulation was taken and dissolved in 50 ml of phosphate buffer pH 6.8. 1 ml, 1.5 ml, and 2 ml of the aforementioned solutions were taken, and 10 ml of pH 6.8 phosphate buffer was added to create 10, 15, and 20 ug/ml solutions, respectively. With the aid of a UV-visible spectrophotometer, the absorbance of each solution was determined at 246.40 nanometers.
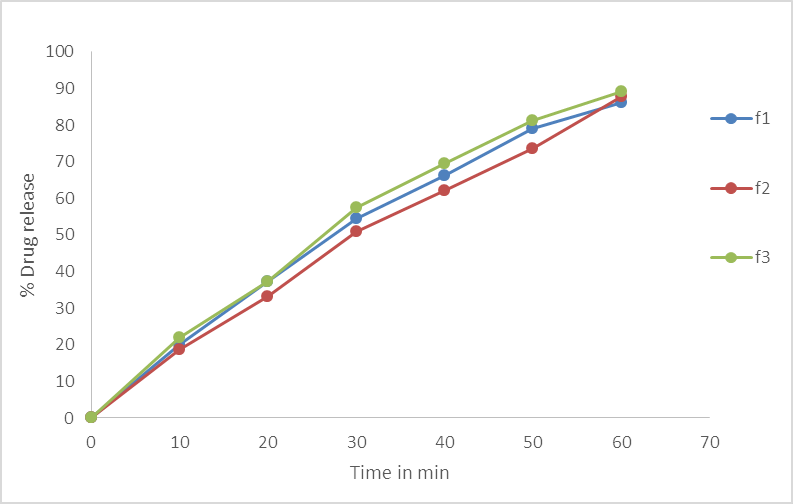
Fig.No.5. % Cumulative Drug Release Of Batch F1, F2, F3.
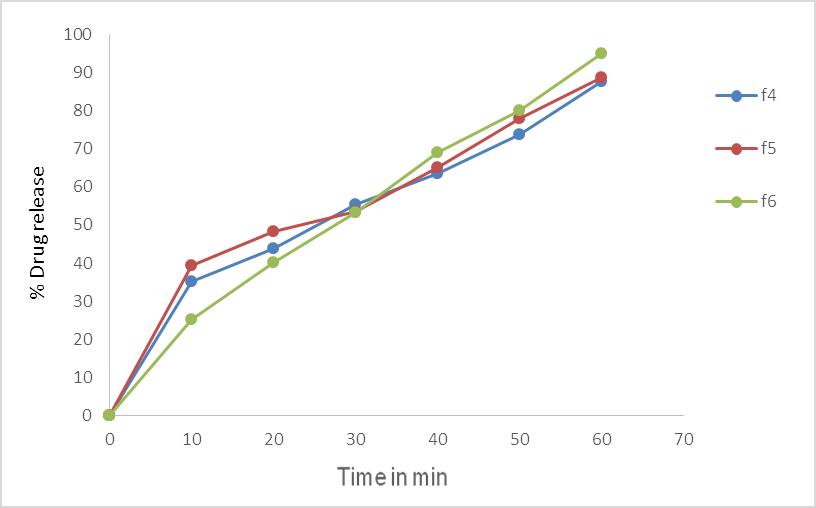
Fig.No.6. % Cumulative Drug Release of batch F4, F5, F6.
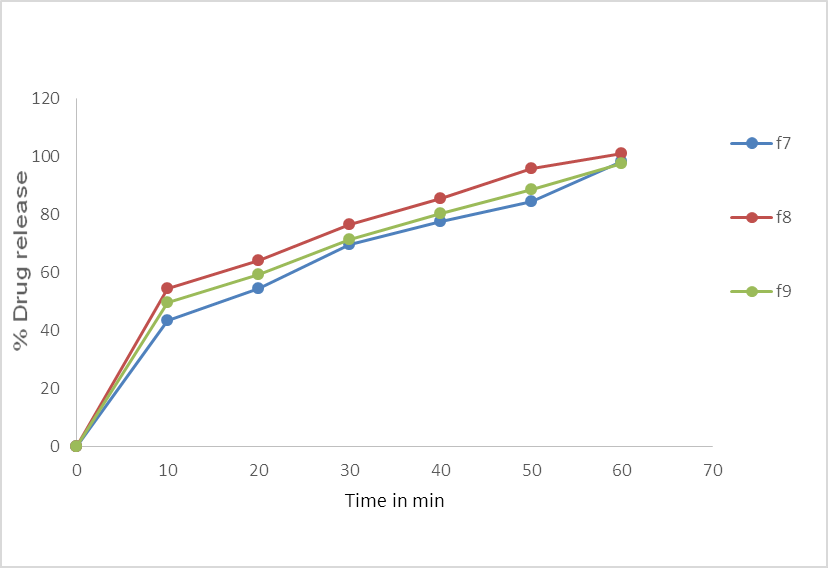
Fig.No.7. % Cumulative Drug Release of batch F7, F8, F9.
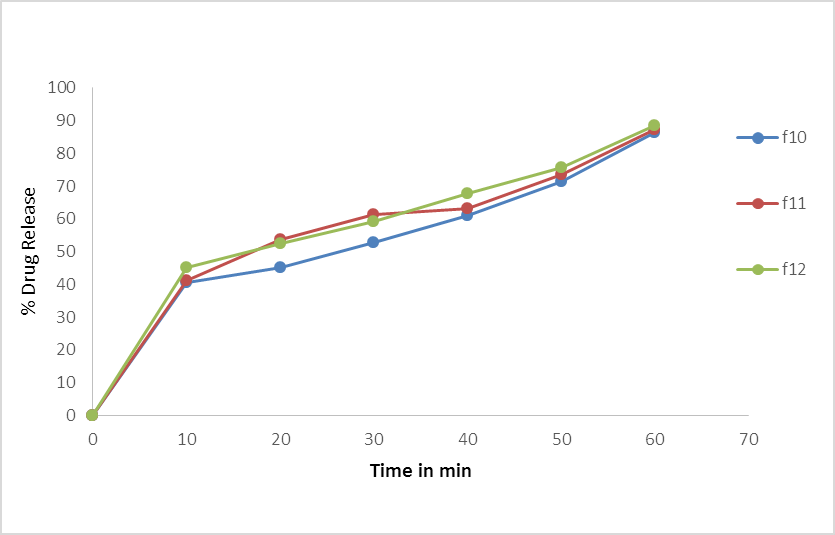
Fig.No.8. % Cumulative Drug Release of batch F10, F11, F12.
|
Formulation code |
% drug content uniformity(%)±S.D |
|
F1 |
97.15±0.6 |
|
F2 |
98.36±0.9 |
|
F3 |
95.21±0.4 |
|
F4 |
97.54±0.7 |
|
F5 |
99.70±0.4 |
|
F6 |
97.43±0.5 |
|
F7 |
99.43±0.2 |
|
F8 |
100.60±0.4 |
|
F9 |
103.76±0.5 |
|
F10 |
99.6±0.3 |
|
F11 |
98.43±0.2 |
|
F12 |
99.55±0.5 |
Table. No.2. Study of % drug content in batches.
Conclusion
According to the study, the medication Montelukast in jelly form is effective since it helps relieve allergy symptoms like sneezing and itching quickly. Patients can use this jelly easily because it can be swallowed and chewed without the need for water. The jelly is not sticky, has a nice consistency, and is free of sugar crystals that could make it difficult to chew. The pH of the jelly, which is almost neutral, influences both its flavor and stability. Even though the jelly is robust and elastic, a lot of force can cause it to rupture and flow. The jelly should spread readily in the mouth as its thickness decreases. The jelly\'s weight varies slightly, but at the tested temperature, there was no evidence of de-swelling. Children should be able to tolerate the flavor, as it was determined to be only mildly to very slightly bitter. It was discovered that one of the formulations (FJ2) had 99.12% of the medication. Throughout testing, the medication was released in varying percentages from 58.52% to 92.08% by each formulation. More medicines were released from formulations containing a low dose of the gelling agent (FJ1 & FJ2). It was discovered that the drug\'s release did not depend on its concentration. A study of a medicated oral jelly containing trazodone hydrochloride was conducted. This medication dissolves readily in water but less readily in ethanol, methanol, and chloroform. Its melting point is 228°C. Tests revealed that the medication and the other chemicals in the jelly got along well. The jellies were created by heating and congealing them, and their look, stickiness, pH, stability, ease of spreading, amount of medicine released, and homogeneity of content were all evaluated. In these tests, the medicated jelly\'s batches F1 through F8 all produced findings that were satisfactory. With a mixture of xanthan gum and gelatin, the F7 batch exhibited 98.95% drug release. Therefore, it was discovered that Trazodone HCl and Montelukast gels were both simple and effective for patient to take.
References
[1] Grodsky GM, Fanska R, Epstein GH, Karam JH. Action of sulfonylureas on the pancreas. Federated Press, 1977; 36: 2714–2719. [2] The 2005 Tripathi KD. Jaypee Brothers Medical Publisher, New Delhi, has published \"Essentials of Pharmacology,\" 5th edition. [3] Kottke MK, Danish M 2007. In Banker G and Rhodes CT, the pediatric and geriatric aspects of pharmacy are discussed contemporary pharmacy. Marcel Dekker Inc., New York. [4] Gohel MC, Parikh RK, and Mohapatra A. Part II: Oral soft gel formulation development and assessment of a patient-friendly metformin hydrochloride dose form. (2008) Asian J Pharm; 2: 172-176. [5] Parikh RK, Gohel MC. Dabhi MR, Nagori SA. Making and assessing the paracetamol-containing soft gellan gum gel. 2009; Indian J Pharm Sci; 71:120–124. [6] Miwa Y, Endo M, and Ishibashi M. formulation of gel for oral use. US Patent No. A1.2008 July 25; US2008/0160087. [7] Hamamoto H, Hirata A, Yokizaki H. Jelly preparation containing biguanide medication March 13, 2007; US Patent No. US2007/0053939A1. [8] Vetrichevlan T, Sundarmoorthy K, Aitha S, and Ayappan T. Levocetrizine hydrochloride mouth dissolving tablets: formulation, optimization, and in vitro assessment for the management of allergic rhinitis. 2010; J Pharm Sci Res; 2: 555–561. [9] Suneela G. and Jeevana JB. Creation of glibenclamide fast-dissolving tablets with crospovidone and kneading mixture. Indian (2010) J Pharm Educ Res 44: 334–340. [10] Mehta T, Patel M, Jogia H. Assessment of dissolving media with a new synthetic surfactant by in vitro testing of BCS class II medications. 2009 J Dissolution Technologies. [11] Mouth dissolving tablets II: an overview of evaluation approaches, Mishra B, Shukla D, Chakraborty S, Singh S. Sci Pharm 2009; 77.
Copyright
Copyright © 2024 Prof. Surywanshi. A. D , Hudge. S. J, Devshatwar. R. S, Chavan. S. I, Kotsulwar. S. S, Salunke. K. J . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64072
Publish Date : 2024-08-24
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

