Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Oral Leukoplakia: Present Views on Diagnosis, Management, Communication with Patients
Authors: krushna Dattu Sangale, Rushikesh Sayaji Thube , Prof. Radhika Kotame, Prof. Khushali Deshmukh
DOI Link: https://doi.org/10.22214/ijraset.2024.65225
Certificate: View Certificate
Abstract
Leukoplakia is a mostly white oral mucosal lesion that has a higher chance of developing into cancer.The review\'s three main goals are to: (1) shed light on the challenges associated with making an accurate diagnosis; (2) assess the most recent findings regarding (bio) markers that may be useful in predicting the likelihood of a malignant transformation; and (3) assess the most recent findings regarding the best course of treatment for patients with oral leukoplakia. Current Research Results The current state of research on oral leukoplakia is very limited, primarily due to the inadequate definition. There are currently no novel (bio)markers that can accurately forecast the onset of malignant transformation. Treatments, both surgical and nonsurgical, have not yet been shown to be successful in stopping such metamorphosis. Synopsis Randomized controlled research on the many elements of oral leukoplakia can only be carried out if the definition\'s flaws and the lack of uniform reporting problem have been resolved.
Introduction
I. INTRODUCTION
With an incidence of more than 300,000 cases worldwide each year, oral cancer is the most prevalent type of cancer of the head and neck. Less than 50% of people who have the disease survive five years, making it a significant source of morbidity and death.1, 2 Early diagnosis of leukoplakia, which is thought to be the most prevalent premalignant oral lesion in the oral cavity and is present in 60% of patients with oral squamous cell carcinoma, is one of the novel strategies for controlling this cancer.3. Such lesions are another indicator of oropharyngeal cancer risk.4
II. DEFINITION OF LEUKOPLAKIA
The World Health Organization (WHO) defines leukoplakia as a clinical entity that is characterized as “a white patch or plaque that cannot be characterized clinically or histologically as any other disease.”5. The fact that it cannot be eliminated by simple scraping completes the definition in everyday practice and sets it apart from pseudomembranous candidiasis. Six Oral leukoplakia affects 0.4% to 0.7% of the population on average. Its incidence is higher in people who are frequent smokers or drinkers, and its distribution by sex is variable.7.
III. LEUKOPLAKIA AND SMOKING
A potent carcinogen, tobacco smoke is thought to be the primary risk factor for head and neck cancers, along with chronic alcoholism.1, 2 As a result, it's important to distinguish between oral leukoplakia in smokers and the so-called "smoker's palate," or stomatitis ni After quitting smoking for four to six months, the lesions completely resolve, demonstrating this causal link. A fingerprint-like or pumice-like pattern, which is a tiny, white striation on the surface that resembles a fingerprint, is a characteristic feature of this clinical presentation.12
Tobacco smoke is the direct causative agent of stomatitis nicotina a clinical condition marked by white lesions on the floor of the mouth or the surface of the soft palate11 (Table 1). Usually, a "Christmas tree," "bell tower," or v-shaped keratinization pattern is shown during histological analysis.
These lesions, which are generated by the proliferative activity of tobacco smoke through the activation of epidermal growth factor receptor (EGFR), which then activates cyclin D1.12, have little potential for malignancy. Nonetheless, prolonged exposure to this carcinogen may raise the incidence of mutations in the oral mucosa and indirectly lead to genomic instability6, which shows up clinically as a lesion that does not resolve after quitting smoking and a loss of fingerprint pattern. In these situations, the plaque can meet the criteria for leukoplakia as stated above.
A. Leukoplakia and Candida
Whether Candida species are involved in the development or etiology of leukoplakias is a topic of significant discussion. Various species of Candida (other from the common albicans variation) that produce nitrosamines have been identified from leukoplakias that are clinically nonhomogeneous and exhibit histological dysplasia.13, 14 Eliminating this surface mycosis does not heal the lesion, but it does cause the high-risk nonhomogeneous leukoplakia variation to change into a low-risk homogenous type. For this reason, fungus superinfection is regarded as a major oncogenesis risk factor.15
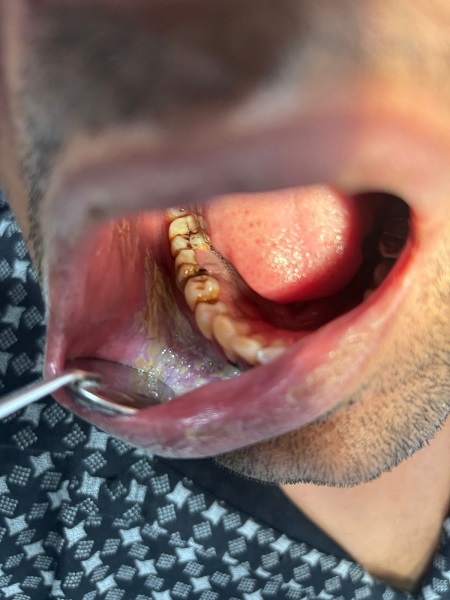
IV. METHODS
Phase 2 trial, open-label, single-group, Dana-Farber Cancer Institute, Boston, MA; trial protocol in Supplement 1. Eligible patients were those with high-risk oral leukoplakia, defined by any of the following criteria: PVL with 4-quadrant oral cavity involvement; at least one localized leukoplakia with moderate dysplasia; or erythroleukoplakia for which surgery was indicated, but not feasible, or a single lesion 4 cm or greater in largest diameter (2-3-4 rule) with epithelial dysplasia (any degree); patient's refusal.
A. Study Population
According to the eighth edition of the American Joint Committee on Cancer Staging Manual, patients who were 18 years of age or older and had an Eastern Cooperative Oncology Group performance status of 2 or lower were allowed to have a history of early-stage oral squamous cell carcinoma (OSCC) or surgically treated carcinoma in situ (CIS) (stages I or II). Participants self-identified their race and ethnicity. which was assessed because the disease's epidemiology and the regional participant demographics of the treatment center could lead to differences in how the study's findings are interpreted if the majority of participants were from one race or ethnic group. (18-387), and was registered across the country (NCT03692325). The Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline was adhered to in this investigation.
B. Treatment
Participants received nivolumab (480 mg intravenously) on day 1 of a 28-day cycle for 4 cycles after providing written informed consent. Immunosuppressive drugs and corticosteroid dosages higher than 20 mg of prednisone equivalent per day were not allowed unless they were utilized to treat immune-related toxicity.
C. Assessments
Patients had digital intraoral color photography at monthly visits and three weeks before the first dosage of nivolumab in order to record all leukoplakia lesions. One of five oral medicine investigators made the determination that up to three target lesions (per patient) may be measured in two dimensions.For uniformity, the oral medicine investigator performed both the screening and posttreatment biopsies.
All target lesions required fresh tissue samples at baseline and 30 days following the last nivolumab dosage. Two skilled oral pathologists (V.Y.J. and K.S.W.) blinded to the outcome data analyzed the pathologic specimens from each biopsy (or a third in the event of any score discrepancy). Rebiopsy may be necessary at any time in the event of new or suspected nontarget lesions or changes to target lesions.A modified composite scoring system (van der Waal classification)19 was used to evaluate the response (eFigure 1 in Supplement 2). A composite score was produced by adding the target lesion point scores, both pathologic and clinical. The best overall response was found by calculating the percent change in the composite score before and after therapy. A drop of more than 80% was considered a major response (MR), a decrease of 40% to 80% was considered a partial response (PR), neither an MR nor a PR was considered stable disease (SD), and a 10% increase in the composite score or a diagnosis of CIS or OSCC was considered a progression of disease (PD). Clinical exams were performed on the patients every three to four months until the end of the trial or for a maximum of five years.
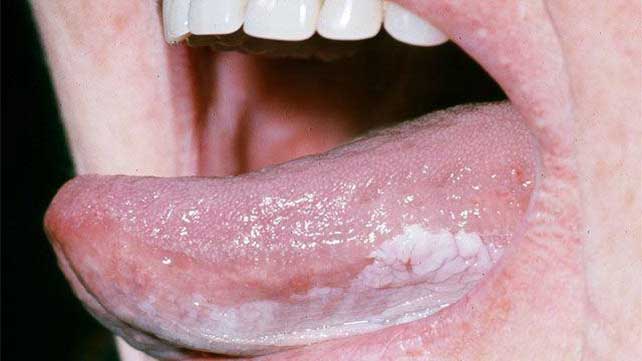
D. Safety
Laboratory and adverse event (AE) assessments were part of the safety evaluations (National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0).20 Nivolumab treatment may be stopped, halted, or postponed for individuals who experienced grade 3 or unacceptable grade 2 immune-related adverse events (irAEs); withdrawal of nivolumab was necessary for specific grade 4 irAEs. AEs were recorded for up to three months following the conclusion of nivolumab therapy.
V. EPIDEMIOLOGY
The most common and highly potential premalignant disorder of the oral cavity is leukoplakia, which has a well-documented epidemiology worldwide. Leukoplakia's incidence varies throughout different scientific research, races, and ethnic groups. With a malignancy conversion rate ranging from 0.12% to 17.51% and a prevalence of 2.65%, it has a thorough global review point.
Based on statistical analysis of multiple Indian studies, the ranges for leukoplakia prevalence are 0.21% to 5.22% and 0.13% to 10% for malignant transformation. Leukoplakia's sharp rise in occurrence, especially in India, may be mostly caused by the country's unique cultural, racial, and geographic characteristics.9.According to research by Downer and Petti, there are between 6.2 and 29.1 occurrences of leukoplakia per 100,000 individuals that have the malignant conversion incidence rate per year. In a different study by the authors, Feller et al. estimated the prevalence of leukoplakia to be closer to 0.5% to 3.46%, whereas Martorell-Calatayud et al. found it to be between 0.4% and 0.7%.9. Additionally, the same study found that leukoplakia had a malignant transformation rate ranging from 0.7% to 2.9%. Another study by Brouns et al. revealed that the yearly malignant transformation rate is 1% and the prevalence is 2%.9. With advancing age, the prevalence rises.9. The claimed incidence often falls between 0.2% and 5%, with notable variations in different parts of the world, such as India (0.2-4.9%), Sweden (3.6%), Germany (1.6%), and Holland (1.4%).10
VI. ETIOLOGY AND RISK FACTORS OF ORAL LEUKOPLAKIA
There are several known and recognized causes of oral leukoplakia, including tobacco use, improperly fitting dentures, bacterial infections, the Epstein Barr virus (EBV), Candida species, and certain herbal plant extracts.
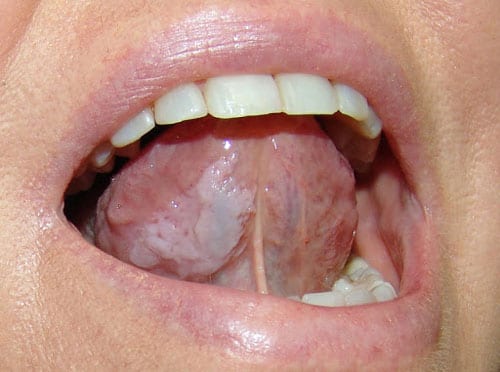
A. Genetic Causes
Each of these incredibly uncommon causes has unique signature traits pertaining to that individual lesion in terms of histopathology.In cases of hereditary benign intraepithelial dyskeratosis, the conjunctiva may develop gelatinous plaques without skin involvement, and the oral mucosa may exhibit bilateral thick white plaques.11, 12 While leukoplakia and oral cancer are brought on by dyskeratosis congenita, both conditions also induce oral plaques, albeit always in the presence of thicker skin lesions.13, 14,
B. Local Injury
Up to 90% of people have leukoedema, which can be brought on by exposure to slightly irritating substances including too much toothpaste, too much mouthwash, and marijuana or tobacco smoke.15, 16, Histopathology reveals only edema of epithelial cells. It manifests as thin, gray-white lacy lines on the ventral tongue or buccal mucosa that vanish when the mucosa is stretched.
C. Tobacco Smoking
Smoking is the most common cause of leukoplakia of the oral cavity mucosa and is a practice that is carried out in many forms throughout the world. Numerous writers have demonstrated that the chemical carcinogens in tobacco cause a range of oral lesions. Smoking is thought to be the cause of leukoplakia in more than 80% of patients who present clinically.17. Oral leukoplakia can develop as a result of both reverse and regular smoking.
D. Fungal Etiology
The oral cavity mucosa near the commissure of the lips is the most frequently affected site in which the shape of the lesion is usually triangular tapering and customarily it is associated with angular chelitis.17 Candida albicans is a normal inhabitant in the oral cavity, throat, large bowel, and vagina. The infection rate increases in pregnancy, tobacco smoking, denture wearers, and presence of any medical conditions or immunosupression.
E. Epstein Barr Virus
Although it can sometimes happen to people who are not HIV positive but are receiving immunosuppressive treatment, Hairy Leukoplakia (HL) is a distinct disease entity that has a strong correlation with HIV infection. The clinical manifestation of Epstein Barr Virus (EBV) is a broad white patch with folds and a corrugated or hairy surface that causes oral leukoplakia. The lesion may be smooth on the surface and visible on other areas of the oral mucosa.17.
F. Bacteria
A little spirochete known as Treponema pallidum is the culprit behind the venereal disease syphilis. Syphilis is primarily spread through sexual intercourse, although it can also spread through blood transfusions and direct touch with an infected person. The germs move quickly through the bloodstream after they penetrate the skin, becoming widely distributed long before any local symptoms show.
G. Vitamin A, B complex, C, E and Beta-carotene
A diet lacking in essential vitamins, minerals, and trace elements can increase the chance of developing leukoplakia.
Sanguinaria Known as sanguinaria-associated keratosis, this kind of leukoplakia typically develops on the maxilla's alveolar mucosa or in the vestibule. The mouthwash and toothpaste contain an extract from herbs, which is the reason of this.
H. Alcohol
While leukoplakia has not been linked to it, it appears to have a high colloborative effect with tobacco in terms of oral cancer formation. Pseukoplakia, or grayish buccal mucosal plaques, are the result of excessive usage of mouth rinses containing alcohol at a concentration higher than 25%. Alcohol makes the oral mucosa more susceptible to the carcinogenic effects of tobacco because it dehydrates it and raises the ambient temperature of the mouth cavity. There are recognized hydrocarbons and nitrosamines in alcohol on its own. A cell's attempt to adjust to alterations brought about by different carcinogens results in increased cell proliferation, cytoplasmic shrinkage, and a rise in the load of related organelles. The earliest sequelae of this oral epithelial proliferation process are hyperplasia and cellular degeneration, which are well-characterized adaptive traits (atrophy) that the epithelium displays when the irritant continues to persist. Apoptosis or malignant transformation are the two stages of irreversible cell damage that the cells eventually reach once the stages of adaptation and reversible cell damage are over.
The accelerated rate of cell division seen in the early phases of transformation is an adaptive response that promotes more genetic damage and drives the cells farther down the path toward malignant transformation.
VII. TREATMENT OPTIONS
- Abolition of tobacco use and monitoring
- Antifungal medications (Candida-related leukoplakia)
- Chemoprevention: Retinoids, Carotenoids, and Vitamins A, C, and E
Topical treatment: bleomycin; vitamin A; photodynamic therapy; surgical excision, with or without grafting; electrocoagulation; cryosurgery; i. laser surgery; and so on.
A. Treatment with Surgery for oral Leukoplakia
1) Conventional Surgery- Excision
The lesion is excised using a scalpel in conventional surgery. [2] In the event of smaller mucosal abnormalities, this is followed by primary closure or secondary healing; in the case of larger defects, local mucosal flaps transposition or even skin grafting may be necessary. [2] If the lesion is large or located in a certain anatomical region, conventional surgery might not be possible. [3] For large lesions, surgery is less desirable due to the related morbidity. [3] As a surgical aftereffect, using a scalpel may result in large regions of denudated mucosa, adverse scarring changes, and secondary functional abnormalities. [4] However, it should be highlighted that curative surgical resection may be useful as a preventative measure for tongue lesions that have a propensity to develop cancer.
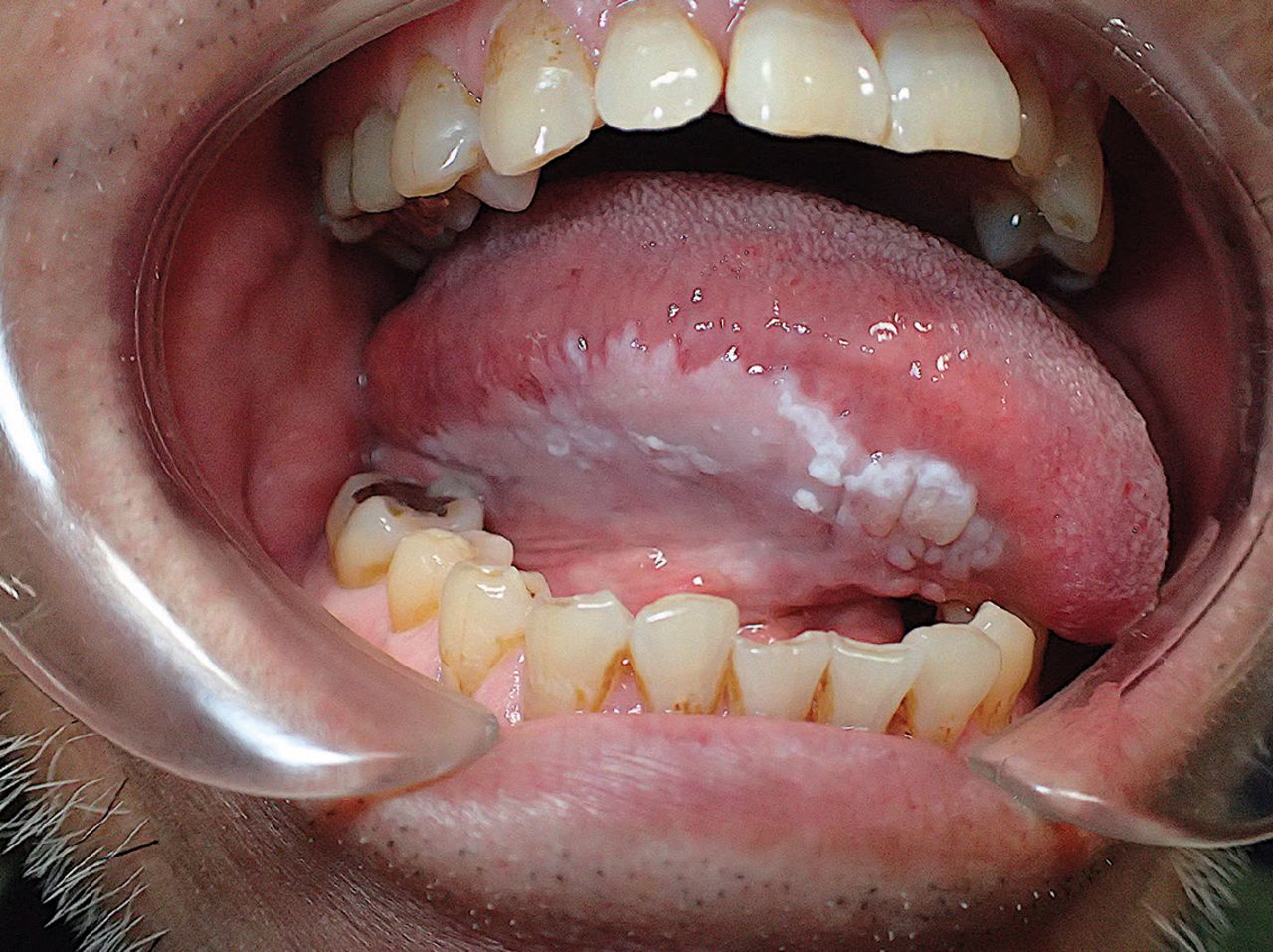
2) Electrocoagulation
Both solo and adjunctive uses of electrocoagulation are possible with scalpel surgery. [2] Thermal injury by electrocoagulation results in postoperative discomfort, oedema, and significant tissue scarring in the surrounding and underlying tissue. Following cryosurgery, oedema and postoperative discomfort are also rather significant. [2, 3]
3) Cryosurgery
One form of treatment called cryosurgery uses low temperatures to deliberately destroy tissue. [1] Using liquid nitrogen (N) or nitrogen dioxide (N2O2), this technique freezes lesional tissue in place [2, 4].[1] In the year 1851, British physician Arnott became the first person to employ cryosurgery. Its application was initially restricted to the management of oral and lip cancer. Cryosurgery is currently being used extensively to treat lesions in the head and neck region, both benign and malignant. [17] Bloodless treatment, a very low rate of secondary infections, and a comparatively painless and scar-free experience are just a few of its benefits. [2, 4] Moreover, the likelihood of newly regenerated epithelium becoming corneous again is decreased [1]
Additionally, patients in high-risk categories such as those with coagulopathies, pacemakers, and the elderly can utilize it. It would also be the first option in situations with numerous, large lesions, locations with challenging surgical access, and situations where aesthetics are crucial.[17]
The success rate of cryosurgery is high, ranging from 80% to 100%. Sufficient freezing depth and freezing time are prerequisites for effectiveness. [1] The depth, size, and form of the pathological lesion, as well as the operator's experience and availability of cryosurgical equipment, all influence the choice of cryosurgical techniques for treating oral leukoplakia. [1] There are two types of cryosurgery apparatus available: open and closed systems. [2, 4, 17] Although closed-system cryotherapy allows more precise temperature control, it necessitates expensive, complicated, and delicate equipment. The cryoprobe is applied directly to the lesional area to complete the procedure.
For the treatment of homogenous, smooth-surfaced oral lesions less than 1 cm in diameter, closed-system cryotherapy is typically appropriate due to the tiny and flat contact area of the cryoprobe end. [4,17] Using a cotton swab or a portable spray device, the cryogen is directly applied to the lesion during open-system cryotherapy. It is more challenging to keep the lesional tissues' temperature consistently lower during the course of treatment. It does not, however, require pricey equipment. The spray device in open-system cryotherapy is appropriate for treating medium- and large-sized oral lesions with a smooth or rough surface. [4]
Both extracellular and intracellular fluids experience ice crystal formation during cryotherapy, which causes cellular dehydration, a hazardous intracellular electrolyte concentration, enzyme inhibition, and protein degradation. Cells undergo the vacuolization, swelling, and eventual rupture caused by these heat shock-related pathways. In addition to the vascular alterations, the treated tissue experiences ischemia necrosis and immunological reactions that result in tissue destruction through cytotoxic immune mechanisms. In [2]
4. Laser surgery (excision or evaporation: According to reports, the procedure that has been most valued over the past 30 years is laser surgery. [/2]
Oral leukoplakia is managed by vaporization or excision using carbon dioxide, potassium-titanyl-phosphate (KTP) lasers, argon, and neodymium: yttrium-aluminum garnet (Nd:YAG). [3]
Conclusion
To a certain extent, oral medicine professionals may find this position paper useful in managing and tracking OLK. According to available data, skilled doctors should oversee and manage OLK patients in order to control the lesion and detect any malignant change early on. All recommendations, however, may need to be modified further when new data becomes available because they are predicated on research that is of poor or extremely low quality. This position paper also emphasizes the critical need for high-caliber multicenter clinical trials on OLK diagnosis and treatment.
References
[1] Classification and nomenclature of abnormalities of the oral mucosa that may be malignant (Warnakulasuriya S, Johnson NW, van der Waal I). Journal of Oral Patholomedica. 2007;36(10):575-80. [2] Van der Waal I, Oral leukoplakia as the focal point, historical perspective, nomenclature of possibly malignant or premalignant epithelial lesions, and recommendations for revisions. In 2018 Oral Surg Oral Med Oral Pathol Oral Radiol; 125(6):577–81. [3] Peterson DE Edwards P, Poh CF, Nikitakis NG, Pentenero M, Georgaki M, et al. Present understanding and potential future implications of molecular markers linked to the onset and development of potentially premalignant oral epithelial lesions. Oral Radiol Surg Oral Med Oral Pathol Oral Surg. 2018;125(6):650–69. [4] Oral premalignant lesions: is a biopsy dependable? Holmstrup P, Vedtofte P, Reibel J, Stoltze K. 2007;36(5):262–265. J Oral Pathol Med. [5] Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7-33; Greenlee RT, Murray T, Bolden S, Wingo PA. [6] Leucoqueratosis nicotínica del paladar; Paricio-Rubio J, Revenga-Arranz J, Ramírez-Gasca T, Boned-Blas P. Dermosifiliogr Actas 2002;93:38–41. [7] Molecular genetics of premalignant oral lesions: Mithani SK, Mydlarz WK, Grumbine FL, Smith IM, Califano JA in the field. In Oral Dis. 2007;13:126–133. [8] Hong WK, Lippman SM. Molecular indicators of oral cancer risk. 2001;344:1323-6; N Engl J Med. [9] World Health Organization: histological typing of cancer and precancerous lesions of the oral mucosa (Pindborg JJ, Reichart P, Smith CJ, Van der Waal I). Springer-Verlag, Berlin, 1997. [10] The Development of Molecular-Based Therapy and Predictive Oncology for Oral Cancer Prevention, Sudbø J, Reith A. Eur J Cancer. 2005;115:339–45. [11] Gintner Z, Dombi C, Bánoczy J. Tobacco use and oral leucoplakia. Journal of Dental Education. 2001;65:322-7. [12] Leffel DJ, Duncan KO, and Geisse JK. tumors of the skin and appendages. Fitzpatrick\'s Dermatology in General Medicine, 7th ed., McGraw Hill, 2007, p. 1024-6, eds. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. [13] Mehta FS, Roed-Petersn B, Pindborg JJ, and Reibel J. Oral leukoplakic epithelium altered by tobacco use. Tumor. 1980;45:2330–6. [14] Mycotic infection in oral leukoplakia, Jepsen A, Winther JE. 1965;23:239–56; Acta Ondontol Scand. [15] Renstrup G. Candida occurrence in dental leukoplakias. 1970;78:421-4; Acta Pathol Microbiol Scand [B] Microbiol Immunol. [16] Reibel J. Molecular biology, histopathology, and clinical features are important in determining the prognosis of oral pre-malignant lesions. 2003;14:47–62 in Crit Rev Oral Biol Med. [17] Tran LM, Dubinett SM, and Krysan K.Regression and immunosurveillance in the context of premalignant squamous pulmonary carcinoma. 10(10):1442-1444 in Cancer Discov. 2020. The doi:10.1158/2159-8290.CD-20-1087 [18] Schepman KP, van der Meij EH, and van der Waal I.An altered scheme for oral leukoplakia staging and categorization. Oral Oncol. 2000; 36(3): 266–267.1016/S1368-8375(99)00092-5 Twenty-one. 15.National Cancer Institute. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 National Cancer Institute; 2017. [19] Magee DE, Hird AE, Klaassen Z, et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors:a systematic review and meta-analysis of randomized clinical trials. Ann Oncol. 2020;31(1):50-60. doi:10.1016/j.annonc.2019.10.008. [20] Oral mucosa variations in look and structure (Canaan TJ, Meehan SC). Dental Clinic North American 2005; 49:1. [21] EJ Raubenheimer, Heyl T. Lip sucking calluses, also known as sucking pads, are a temporary sign of leukoedema in newborns. In 1987, Pediatr Dermatol. 4:123–128. [22] Abdulreddin Nureddin. Leukoplakias are now classified as distinct disease entities and have a new definition. Research in Dental and Oral Health. 2018;7:555717. [23] Snijders PJ, Walboomers JM, Lamey PJ, Cruz I, Napier SS, van der Waal I, et al. High grade dysplasia and the possibility of malignant transformation are closely linked to suprabasal p53 immunoexpression in oral lesions from Northern Ireland that may develop into cancer. Journal of Clin Pathol. 2002;55:98–104. [24] Caldeira PC, Abreu MH, do Carmo MA. Binary system of grading oral epithelial dysplasia: evidence of a bearing to the scores of an immunohistochemical study. J Oral Pathol Med. 2012;41:452-453. [25] Chattopadhyay A, Ray JG. AgNOR cut-point to distinguish mild and moderate epithelial dysplasia. J Oral Pathol Med. 2008;37:78-82. 21. Greenberg MS, Glick M. Burket’s Oral Medicine. Red and white lesions of the oral cavity. Delhi, India: BC decker Inc. Elsevier; 10th ed , pp. 85-125;2005. [26] Skalko-Basnet N, Basnet P.On the road to treating cancer, curcumin—an anti-inflammatory molecule found in curry spices—may be useful. Molecules, 16(6), 4567–4598, 2011 Jun 3. [PubMed][Cross Reference] [27] Shenoy AS, Dessai SR, and Satoskar SK. Oral leukoplakia: photodynamic treatment for management. 2016 Apr-Jun;2(2):99-101 International Journal of Oral Health Dentistry [28] Velpula N, Goyal S, Lingam S, Chappidi V, Kodangal S, and Maloth KN. Photodynamic therapy: A novel approach for treating cancer and precancerous conditions. 2014 Apr;5(4):250–257 in IJCRI. [29] Yelisetty K, Kotha P, Veeraraghavan G, Reddy RS, and Praveen KNS.A review of two cases involving cryosurgery for the treatment of potentially malignant lesions. IJSS Case Reports & Reviews. 1(8):5–9, January 2015. [30] Jerjes W, Hamdoon Z, Hopper C. CO2 lasers in the treatment of oral illnesses that may be malignant or possibly malignant. Head Neck Oncol. Apr 30, 2012; 4:17. [PubMed] [Cross Reference]
Copyright
Copyright © 2024 krushna Dattu Sangale, Rushikesh Sayaji Thube , Prof. Radhika Kotame, Prof. Khushali Deshmukh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET65225
Publish Date : 2024-11-13
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

