Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Recent Advancements in Water Waste Water Treatment Technologies
Authors: Spoorthy R, Sanjana Poojar , Pruthvi H R , Prakruthi YD
DOI Link: https://doi.org/10.22214/ijraset.2024.63963
Certificate: View Certificate
Abstract
Water is undoubtedly the most important and invaluable natural resource that humans utilize. The main source of water for human consumption and various industrial uses on a global scale is groundwater. Different types of organic and inorganic contaminants from natural and, more often, anthropogenic sources, leach into all water systems, especially groundwater. A lack of water for human consumption is also a result of inadequate wastewater treatment, rising freshwater usage, and climate change. Therefore, it is necessary to adequately and conscientiously manage wastewater to ensure safe drinking water and sanitation for all, which is in line with Sustainable Development Goal 6 on water and sanitation (SDG 6). The systematic treatment of wastewater is carried out through three successive stages are primary (physical–chemical), secondary (biological), and tertiary (advanced physical–chemical) treatment. The primary goal of any wastewater treatment plant is to meet the legal regulations for the discharge of treated wastewater into water bodies. Currently, it is very challenging to achieve the required quality of purified wastewater, especially due to the appearance of emerging environmental pollutants. For this reason, advanced knowledge is necessary and desirable in developing new advanced technologies or improving existing technologies at all levels of wastewater treatment.
Introduction
I. INTRODUCTION
Water is one of the essential components required in the growth and survival of human beings as well as all living species. Water is available in huge quantities all over the world, but only about 1% of it is easily available for utilization. As per a new report published by World Health Organization (WHO) in 2015, more than 1.1 billion people are struggling worldwide in order to acquire access to pure drinking water. Exponential population growth and rapidly growing industrialization are two major causes of water pollution. The main purpose to treat wastewater is to mineralize the various complex nondegradable pollutants that are present in various industrial/domestic effluents so that they can be safely discharged and do not contaminate the surface water and ground waters.
The industrial effluents contain various types of organic and inorganic pollutants and are hence required to be treated by various physical, chemical, and biological treatment techniques to obtain water quality within permissible limits as prescribed by the various regulatory bodies.
Conventional biological treatment methods such as aerobic, anaerobic, and facultative processes are available, but they need to be intensified further because these individual processes are incapable of causing complete breakdown of the highly complex biorefractory pollutants present in wastewater. Moreover, chemical methods including adsorption, ion exchange, membrane filtration, and chemical oxidation are also available, but these methods usually generate large amounts of waste sludge and, moreover, require large quantity of chemicals for treating the bulk of effluent.
II. LITERATURE REVIEW
A. Current Perspectives, Recent Advancements, And Efficiencies Of Various Dye-Containing Wastewater Treatment Technologies
Reference: Mohammad Danish Khan, Ankit Singh, Mohammad Zain Khan, Shamas Tabraiz and Javed Sheikh, 2023.
The usage of dyes is increasing due to their high demand in expanding industrial sector. As a result, large volumes of dye wastewater are being generated, particularly in the textile industry. To reduce the detrimental effects of dye wastewater on the environment, it should be treated before its disposal. This article extensively reviews the existing and advancements in physical, physicochemical, chemical, and biological treatment technologies and their efficacies in dye removal (%). Researchers have increasingly focused on bioremediation as an essential method for removing dangerous dye contaminants from natural water bodies. Dye molecules contain chromophores which give them colour, and the auxochromes are responsible for enhancing the dye's colour. The wavelength of light absorbed by the chromophores and auxochromes determines the varied colours that the dyes produce. The two types of dyes available are natural and synthetic. The aim of this article was to review the treatment methods used for various dye removal especially for dye effluent from textile industry and compare their removal efficiency, and outline the operational parameters that influence the treatment processes, and recent advances in dye-containing wastewater treatment. As a result, most of the reported physical, physicochemical, chemical and biological technologies can remove >90 % of dye.
B. Water Treatment Technologies In Removing Heavy Metal Ions From Wastewater
Reference: Tawfik A Saleh, Mujahid Mustaqeem and Mazen Khaled, 2022
In this article, current development in recent waste-water treatment technologies to remove heavy metals has been reviewed and summarized. This review evaluates the state-of-the-art removal processes, including chemical precipitation, photocatalysis, flotation, ion exchange, remediation, electrochemical treatment, adsorption, membrane technologies, and coagulation/flocculation. Numerous factors are responsible for the accumulation of heavy metal ions in wastewater due to various industrial operations such as electrolysis, electroplating, and metal smelting, as well as chemical contaminants in the environment. In addition, heavy metal removal is also a general health concern as they are teratogenic, carcinogenic, and causes detrimental health problems. The chemical precipitation method is widely used due to its simplicity, the membrane filtration process is also effective, however, it requires a large cost for its initial operation, The ion exchange method can exchange anions and cations from solution media but fails in concentrated metals solutions. These processes are pH-dependent and continuous monitoring and controlling the pH is critical. The above-mentioned processes have disadvantages like high cost, partial removal of certain ions, sludge generation, the extra operational cost for sludge disposal, and high working cost due to membrane fouling. In recent years, other alternative treatment methods, such as photocatalysis, electrochemical, flotation, coagulation, and adsorptions attracted great attention.
C. A Critical Review On Diverse Technologies For Advanced Wastewater Treatment During Sars-Cov-2 Pandemic
Reference: Sasan Zahmatkesh , Kassian T.T. Amesho and Mika Sillanpaa, 2022
Advanced wastewater treatment technologies are effective methods and currently attract growing attention, especially in arid and semi-arid areas, for reusing water, reducing water pollution, and explicitly declining, inactivating, or removing SARS-CoV-2. Advanced wastewater treatment processes are highly recommended for contaminants such as monovalent ions from an abiotic source and SARS-CoV-2 from an abiotic source. This work introduces the fundamental knowledge of various methods in advanced water treatment, including membranes, filtration, Ultraviolet (UV) irradiation, ozonation, chlorination, advanced oxidation processes, activated carbon (AC), and algae. Thus, for membrane-based treatments, the membrane size determines the maximum pore size of the membrane, which must then be selected in order to remove viral particles. As a result, in order to inactivate SARS-CoV-2, UV radiation must also be intense enough to penetrate RNA and DNA. In addition, various oxidizing radicals are created during chlorination, which aids in inactivating DNA, thereby inactivating SARS-CoV-2.
D. Advancement In Treatment Of Wastewater With Nano Technology
Reference: Komal P. Mehta, Rajneesh Sharma, Shubhajit Haldar and Atul Kumar, 2023
In this paper overview of nanotechnology application for waste water treatment is presented. By understanding characteristics and applying its use in advancement for existing treatment methods, sustainability can be increased to supply good quality of water to people. For removing residual pollutants and microbial pathogens, advanced oxidation process such as Photocatalytic oxidation is in process. Photocataytic oxidation enhances biodegradability as it can remove hazardous and non biodegradable pollutants. It can be used as a first step for removal of organic compound. Main problem is kinetics because of limited light fluency and photocatalytic activity.
Till date most difficult task in the handlingof water & waste water is lack of ability to detect fast growing pathogens, lower concentration of some pollutants, toxicity, complexity of pollutants in water / waste water. With characteristics of sensitivity and selectivity innovative sensors can be designed to get fast response which may be utilized for fast treatment. Application of nano materials, its actual applications will be as non absorbent, nano technology enabled membranes and nano photo catalysts. Due to unique characteristics of nano materials and possibility of its combination with existing treatment in water/ waste water treatment, its acceptance is increased world.
E. Recent Advances In The Biological Treatment Of Wastewater Rich In Emerging Pollutants Produced By Pharmaceutical Industrial Discharges
Reference: A.khalidi-idrissi, A.Madinzi, A.Anouzla, A.Pala, L.Mouhir, Y.Kadmi and S.Souabi, 2023
Pharmaceuticals and personal care products present potential risks to human health and the environment. In particular, wastewater treatment plants often detect emerging pollutants that disrupt biological treatment. The activated sludge process is a traditional biological method with a lower capital cost and limited operating requirements than more advanced treatment methods. In addition, the membrane bioreactor combines a membrane module and a bioreactor, widely used as an advanced method for treating pharmaceutical wastewater with good pollution performance. Indeed, the fouling of the membrane remains a major problem in this process. In addition, anaerobic membrane bioreactors can treat complex pharmaceutical waste while recovering energy and producing nutrient-rich wastewater for irrigation. Hybrid systems can generate bioenergy, which helps reduce the operating costs of the pharmaceutical waste treatment system. Water in the pharmaceutical industry must be controlled both upstream and downstream of the plant: upstream, water must be saved by rationalizing and recycling treated water. Downstream, all types of pollution must be treated before discharge into the environment, with recycling of treated water. For downstream actions, among the biological treatments recommended for pharmaceutical waters, we can mention membrane processes, biological treatment by activated sludge, techniques using rotating disks as biomass support, membrane bioreactors with high antibiotic reduction efficiency, anaerobic treatment techniques alone or combined with aerobic treatment.
F. Integration Of Electrocoagulation Process With Submerged Membrane Bioreactor For Wastewater Treatment Under Low Voltage Gradients
Reference: Khalid Bani-Melhem, Maria Elektorowicz, Muhammad Tawalbeh, Abeer Al Bsoul, Ahmed El Gendy, Hesam Kamyab and Mohammad Yusuf, 2023
The study aimed to address membrane fouling in the submerged membrane bioreactor (SMBR) used for wastewater treatment. An aluminum electrocoagulation (EC) device was combined with SMBR as a pre-treatment to reduce fouling. The EC-SMBR process was compared with a conventional SMBR without EC, fed with real grey water. To prevent impeding biological growth, low voltage gradients were utilized in the EC device. The comparison was conducted over 60 days with constant transmembrane pressure and infinite solid retention time (SRT). In phase I, when the EC device was operated at a low voltage gradient (0.64 V/cm), no significant improvement in the pollutants removal was observed in terms of colour, turbidity, and chemical oxygen demand (COD). Nevertheless, during phase II, a voltage gradient of 1.26 V/cm achieved up to 100%, 99.7%, 92%, 94.1%, and 96.5% removals in the EC-SMBR process in comparison with 95.1%, 95.4%, 85%, 91.7% and 74.2% removals in the SMBR process for turbidity, colour, COD, ammonia nitrogen (NH3–N), total phosphorus (TP), respectively. SMBR showed better anionic surfactant (AS) removal than EC-SMBR. A voltage gradient of 0.64 V/cm in the EC unit significantly reduced fouling by 23.7%, while 1.26 V/cm showed inconsistent results. Accumulation of Al ions negatively affected membrane performance. Low voltage gradients in EC can control SMBR fouling if Al concentration is controlled.
G. The Coupling Of Anammox With Microalgae-Bacteria Symbiosis: Nitrogen Removal Performance And Microbial Community
Reference: Jiannv Chen, Xiangyin Liu, Tiansheng Lu, Wenxuan Liu, Zhiwen Zheng, Wenxi Chen, Chu Yang, Yujie Qin, 2024
The partial nitrification-anammox process for ammonia nitrogen wastewater treatment requires mechanical aeration to provide oxygen, which is not conducive to energy saving. The microalgae-bacteria symbiotic system (MaBS) has the advantages of low carbon and energy saving in wastewater biological nitrogen removal. Therefore, this study combined the MaBS with an anammox process to provide oxygen, through the photosynthesis of microalgae instead of mechanical aeration. They investigated that the nitrogen removal efficiency and long-term operation of a co-culture system comprising microalgae, nitrifying bacteria (NB), denitrifying bacteria (DnB), and anaerobic ammonium-oxidation bacteria (AnAOB) in a sequencing batch reactor without mechanical aeration.
The experiment was divided into three steps: firstly, cultivating NB; then, adding three kinds of microalgae which were Chlorella sp., Anabaena sp., and Navicula sp. to the bioreactor to construct a microalgae-bacteria symbiotic system; finally, adding anammox sludge to construct the anammox and microalgae-bacteria symbiosis (Anammox-MaBS) system. The results demonstrated that nitrification, denitrification, and anammox processes were coupled successfully, and the maximum TN removal efficiency of the stable Anammox-MaBS system was 99.51 % when the concentration of the influent NH4+-N was 100 mg/L without mechanical aeration and organic matter addition.
H. Regulation Of Extracellular Polymers Based On Quorum Sensing In Wastewater Biological Treatment From Mechanisms To Applications
Reference: Longyi Lv, Li Sun, Ziyin Wei, Weiguang Li, Jiarui Chen, Yu Tian, Wenfang Gao, Pengfei Wang, Zhijun Ren, Guangming Zhang, Xiaoyang Liu and Huu Hao Ngo, 2024
The applications research on improving wastewater biological treatment performance based on QS regulated EPS have been widely reported, but reviews on the level of QS regulated EPS to enhance EPS function in microbial systems are still lacking. Extracellular polymeric substances (EPS) regulated by quorum sensing (QS) could directly mediate adhesion between microorganisms and form tight microbial aggregates. Besides, EPS have redox properties, which can facilitate electron transfer for promoting electroactive bacteria. By synthesizing the role of QS in EPS regulation, they further point out the applications of QS-regulated EPS in wastewater biological treatment, which involve a series of aspects such as strengthening microbial colonization, mitigating membrane biofouling, improving the shock resistance of microbial metabolic systems, and strengthening the electron transfer capacity of microbial metabolic systems. QS regulates EPS synthesis by regulating the expression of genes in the ATP synthesis and carbon metabolism pathways to provide sufficient energy and substrate material for the synthesis of EPS. In microbial metabolic systems, QS signaling molecules are significantly associated with EPS secretion. Thus, EPS that are based on QS-regulated can enable performance optimization of wastewater biological treatment systems, such as; microbial colonization, mitigation of membrane contamination.
III. INNOVATIONS IN WASTEWATER TREATMENT
A. Membrane Bioreactors (MBRs)
The need for an excess supply of water and the generation of high effluent quality upon proper treatment technologies has become a necessity. These two crucial needs can be achieved with the aid of membrane bioreactor (MBR) that has been proven to be effective in removing organic and inorganic matters as a biological unit for wastewater treatment. MBR plants are created by integrating the biological process with membrane filtration which possesses numerous benefits if compared with conventional methods such as activated sludge; MBR is widely used for municipal and industrial wastewater treatment. The characteristics of the bioreactor treatment process is discussed in detail, and then a comprehensive review of the membrane separation process is examined. The fouling phenomena as a main obstacle to widespread MBRs plant is presented in detail with recent fouling mitigation methods. The efforts of a number of novel MBR processes are summarized.
B. Biological Wastewater Treatment
The bioreactor operating conditions highly influence such characteristics of the microorganism as size, content of filamentous microorganisms, growth rate, etc. On the other hand, the activity of microorganisms can affect the performance of the MBR in two different ways; in the quality of the effluent and how much the MBR can treat the wastewater pollutant, and the fouling properties of the membranes. Hence, profound study of the principle of the biological wastewater treatment such as microbiology, metabolism of microorganism, microbial stoichiometry, and kinetics in bioreactor is necessary in order to determine the optimum operating conditions of the bioreactor and design characteristics of the MBR plants. Due the influence of wastewater from the atmosphere, which is fed into the bioreaction, diverse microorganisms are structured into variety of communities. However, by adjusting operating conditions and reactor design, specific type of microorganisms can be enriched in the bioreactor.
C. Membrane Fouling in MBR
Fouling in MBRs occurs in different forms, namely, pore narrowing, pore clogging and, cake formation. Pore clogging refers to the blocking of membrane micro pores by foulants. Pore clogging depends, to a large extent, on the size of the particle and the membrane pore size. The attachment of the materials in the pores is aided by sticky substances in the solution. Cake formation, on the other hand, results from the continuous accumulation of bacteria clusters, biopolymers and inorganic matter, which form a layer (biocake) on the membrane.
The cake layer increases membrane filtration resistance. Membrane fouling mechanisms in MBRs are schematically illustrated below.
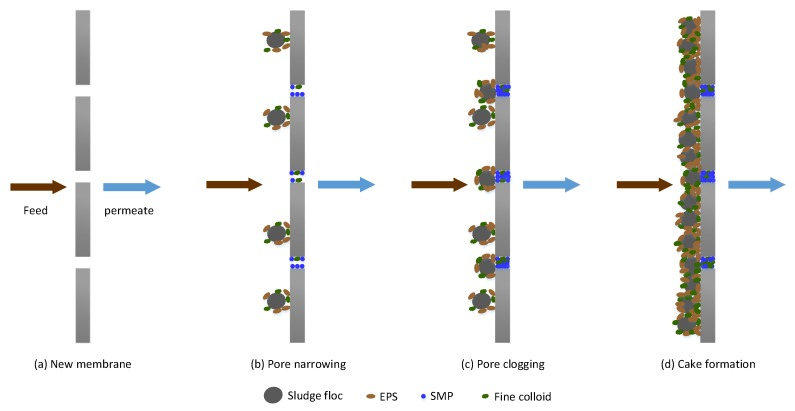
Mechanisms Of Membrane Fouling In MBR
Membrane fouling decreases the permeate flux when the MBR is operated at constant transmembrane pressure (TMP), and results in the increase of TMP when the MBR is operated at constant permeate flux. At constant flux operation, a sharp increase in TMP indicates severe membrane fouling. This sudden TMP increase is called a “TMP jump”. The objective of fouling control is to retard TMP jump through modification of sludge characteristics (MLSS, floc size, EPS content, and apparent viscosity) or lowering of operational flux.
D. Classification Of Foulants
Biofoulants: refers to the bacteria or flocs whose deposition, growth and metabolism on the membrane results in fouling. For a start, one bacteria cell may attach to the membrane surface or inside its pores and, after some time, the cell multiplies into a cluster of cells, leading to the formation of biocake, and hence reduced permeability. The bacteria (biofoulants) and their metabolic products contribute to fouling. Essentially, membrane biofouling is a two-step process, starting with early bacterial attachment, followed by multiplication of bacteria on the membrane surface.
Organic Foulants: refers to biopolymers, e.g., polysaccharides and proteins, of which deposition on the membrane results in a decline of membrane permeability. These foulants are found in metabolic products of bacteria, which are collectively called EPS. Compared to large particles, such as sludge floc, the deposition of organic foulants on the membrane surface is more difficult to remove.
E. Types of MBR
Aerobic and anaerobic membrane bioreactor (AnMBR)
To treat wastewater and effluent from industries, aerobic treatment technology has been utilized for a century. However, high energy demand for aeration process, generating high amount of sludge, emission of greenhouse gases such as nitrous oxide, large footprint and high maintenance cost are main drawbacks of such technology. For wider application of aerobic MBR, reduction of required energy is crucial where aeration control strategies in aeration tanks plays a significant rule to deduct overall energy consumption of the process. In full scale MBRs, recent studies showed reduction in aeration and energy consumption rate of 20% and 4%, respectively, by employing ammonia-N-based aeration control strategy.
An alternative MBR configuration has been developed by integrating anaerobic digestion treatment with membrane filtration to treat wastewater and overcome some obstacles of MBR processes. In this case, the energy requirement of wastewater treatment is reduced during through the decomposition of organic matters to methane-rich biogas. Additionally, nutrient recovery is possible with following precipitation due to converting nutrients into chemically available forms. However, membrane stability, membrane fouling, dilute resources, and salinity built-up are some of the main challenges that prevents its development
Anaerobic bioreactor and membrane models composed the two parts of AnMBR plant. In terms of bioreactor configurations, up-flow anaerobic sludge blanket (UASB), completely stirred tank reactor (CSTR), and anaerobic fluidized bed bioreactor (AFBR) are the most frequent ones for AnMBR with the CSTR being the most commonly structure that is used in AnMBR due to simple operating and construction process.
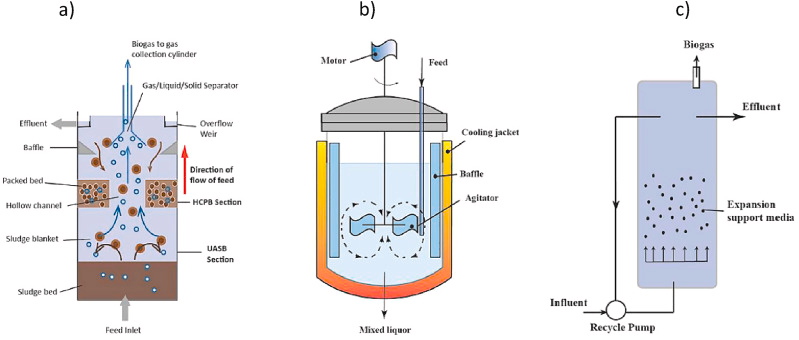
Typical anaerobic bioreactors a) up-flow anaerobic sludge reactor; b) continuous stirred-tank reactor; c) anaerobic fluidized bed reactor.
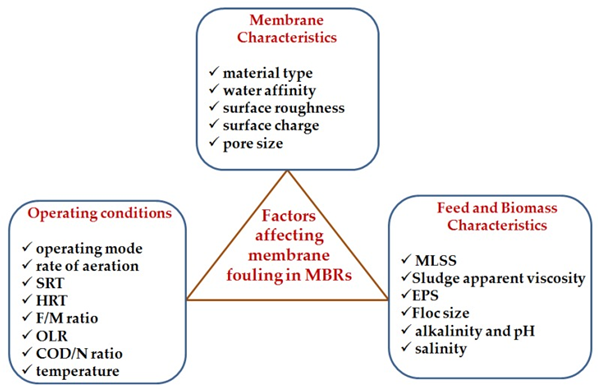
F. Membrane Characteristics
- Membrane Material: The material the membrane is made of has an impact on its fouling propensity in MBRs. Based on the membrane material, membranes can be classified into: ceramic membranes, polymeric membranes, and composite membranes.
- Water Affinity: The water affinity (hydrophilicity or hydrophobicity) property of the membrane material affects fouling in MBRs. The water affinity behaviour of a membrane material is determined by measuring the contact angle of a water drop on its surface. Smaller angles indicate hydrophilicity, whereas larger angles indicate hydrophobicity.
- Membrane Surface Roughness: The surface roughness of the membrane material also has some influence on membrane fouling in MBRs. Membranes with homogeneous surfaces are less subject to be fouled than those with uneven surfaces. Research findings have indicated that membranes with higher surface roughness foul faster.
- Membrane Surface Charge: The membrane surface charge is another property of importance in relation to membrane fouling especially if there are charged particles in the feed. It has been indicated that most membrane materials are negatively charged under normal conditions.
- Membrane Pore Size: Generally, membranes used in wastewater treatment are broadly grouped into two: porous membranes and non-porous membranes. Porous membranes employ straining, sieving, or size exclusion to separate particles, e.g., microfiltration (MF), ultrafiltration (UF), and loose end nanofiltration (NF) membranes. Non-porous membranes, on the other hand, make use of the differences in diffusivity or solubility between the solvent and the solute in the membranes for separation.
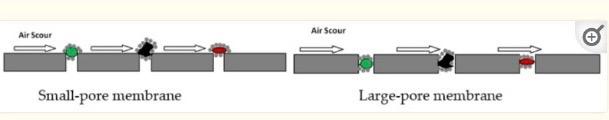
Schematic of pore blocking on small-pore and large-pore membranes.
G. Operating Conditions
- Operating Modes: Are typically used in MBRs namely, constant TMP with variable permeate flux and constant permeate flux (L/m2 h) with variable TMP. The latter is the preferred mode in MBRs as it can readily handle fluctuations in influent hydraulic loading. When operating at constant permeate flux, membrane fouling is observed by TMP jump. The critical flux needs to be determined in constant flux operation, because it is an important parameter in MBR operation.
- Rate of Aeration: aeration plays a dual role in aerobic MBRs. It supplies oxygen for the biological processes and serves as a way of dislodging the cake layer formed on the membrane surface (air scouring). The oxygen supplied via aeration facilitates the biodegradability and cell synthesis of the biomass. Research has shown that increasing the rate of aeration in an MBR leads to a reduction in membrane fouling.
- Solids Retention Time (SRT): The SRT is a very important factor affecting membrane fouling in MBRs. The SRT varies with the formation of EPS. A majority of studies indicate that increasing the SRT results in a decrease in the concentration of EPS as the biomass stays longer in the system, and lowering the SRT increases the amount of EPS. High SRTs produce starvation conditions in the bioreactor creating an enabling environment for reduced formation of EPS, low sludge production and nitrification.
- Hydraulic Retention Time (HRT): The HRT has indirect effect on membrane fouling as it determines, with other operating parameters, the sludge characteristics. Most researchers agree that as HRT decreases, the rate of membrane fouling in MBRs increases due to an increase in sludge viscosity and EPS concentrations. The decrease in HRT stimulates the release of EPS from bacterial cells, causes overgrowth of filamentous bacteria, and the formation of irregular large flocs.
- Food–Microorganisms: (F/M) Ratio The F/M ratio is an important operational parameter in biological wastewater treatment systems. In order to determine the influence of F/M ratio on membrane fouling, Kimura et al investigated fouling in three identical pilot-scale MBRs using real municipal wastewater under different operating conditions. Their results indicated that the F/M ratio influences the nature of the foulants as high F/M correlated with higher proteinaceous foulant.
- Organic Loading Rate (OLR): The OLR is one of the most important parameters affecting the operation of biological wastewater treatment systems. In MBR, Zhang et al investigated the effect of constant and variable influent OLR on membrane fouling using two identical laboratory-scale submerged MBRs operated for 162 days at an SRT of 30 days; the influent OLR was kept constant in one MBR and varied in the other.
- Chemical Oxygen Demand/Nitrogen (COD/N): Ratio The COD/N ratio is one of the most important parameters for the growth of microorganisms. It also plays a key role in nutrients’ removal (particularly nitrification and denitrification). This operating parameter has also been reported to correlate well with membrane fouling in MBRs. Feng et al studied the effect of COD/N ratio on membrane fouling in two identical submerged MBRs operated in parallel at COD/N ratios of 10:1 and 5:1, respectively. They reported that operation at COD/N ratio of 10:1 remarkably reduced membrane fouling rate through the slower rise in TMP compared to the MBR operated at COD/N ratios of 5:1.
- Temperature: Temperature is known to influence the rate of biodegradation. In MBRs, temperature impacts membrane fouling by altering the MLSS (Mixed Liquor Suspended Solids) characteristics. It has been reported that decreasing operational temperature causes the bacteria to release more EPS. Very low temperatures are associated with an increased occurrence of filamentous bacteria, which produce more SMPs in the MLSS, hence more propensity for membrane fouling.
H. Conclusions
Effective fouling prevention methods and proper operation can truly sustain the performance of MBRs. This was determined after several investigations on important aspects of MBRs such as design strategies and fouling phenomenon. This review has summarized each basic concept pertaining to each section of MBRs such as biological bioreactor, membrane modules, membrane fouling phenomena among developments in control strategies.
In terms of the successful curtailing membrane fouling control, fouling itself is a major challenge in the applications of membrane technologies despite of all the efforts that have been done. There are different factors that influence membrane fouling in MBRs. These factors include: membrane characteristics (material type, water affinity, surface roughness, surface charge, and pore size), operating conditions (operating mode, rate of aeration, SRT, HRT, F/M ratio, OLR, COD/N ratio, and temperature). EPS, in particular, are major contributors to membrane fouling.
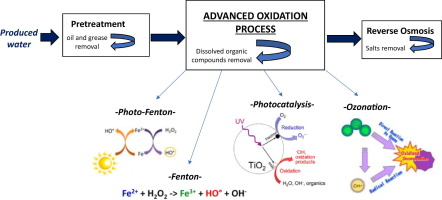
I. Advanced Oxidation Process (AOPs)
AOPs are defined as processes that involve the generation and use of the OH- radicals as a strong oxidant to destroy (oxidize/mineralize) the organic compounds that cannot be oxidized by conventional oxidants such as gaseous oxygen, ozone, and chlorine. The OH- reacts with the dissolved constituents, initiating a series of oxidation reactions until the constituents are completely mineralized to CO2 and H20. The AOP processes include the different oxidation technologies such as cavitation, photocatalytic oxidation, sonolysis, and Fenton chemistry.
J. Hydrodynamic Cavitation
Cavitation process comprises the sequential formation, growth, and subsequent collapse of microcavities within a very short duration, which thereafter causes prolonged physical and chemical effects within the aqueous medium so as to achieve the desired transformation. As a consequence, these cavitational effects include generation of hot spots and high-intensity turbulence, and this resultant energy is subsequently diffused to the molecular level within the liquid, thereby causing the generation of OH- that induce chemical changes in the form of oxidation of pollutants.
HC is simply generated by varying the flow conditions of a liquid by passing it through a constriction fitted in a conduit. The variation of pressure when flowing through these constrictions such as venturi and orifice with different geometries lead to the generation of hot spots or cavities. During HC treatment, the water molecules get dissociated into OH- and hydrogen radicals (H+) under extreme temperature and pressure conditions. These OH- with high oxidation potential oxidize organic pollutant molecules present in the waste water and thereby degrade the organic compounds.
K. Sonolysis Or Acoustic Cavitation
Sonication of water by ultrasonic sound waves having high frequencies ranging from 16 kHz to 2 MHz leads to the formation, growth, and collapse of microcavities through compression and rarefaction cycles, which is known as sonolysis or AC. When adequate negative pressure appears within the liquid, the average molecular distance between liquid molecules becomes higher than the critical molecular distance that bound liquid molecules together, and therefore the liquid molecules breakdown, and cavities are created. These cavities grow in size until the maximum negative pressure has been reached. The growth and collapse of these cavities result in the generation of high pressure, temperature, and highly reactive OH- by thermal decomposition of water and oxygen. In order to obtain higher efficiency, several operational parameters such as intensity of sound waves, frequency of ultrasound, and calorimetric efficiency of ultrasonic equipment need to be optimized. The efficiency of the sonolysis process is also dependent on the solvent properties, for example, vapor pressure, viscosity, surface tension, and density.
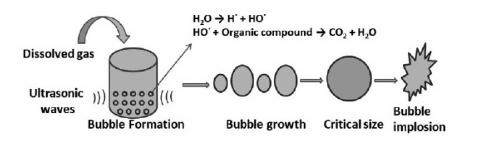
Schematic representation of sonolysis process
L. Photocatalysis
The photocatalytic oxidation process is one of the most important techniques in the area of wastewater treatment. During this process, the solid semiconducting photocatalysts, such as TiO2, WO, ZnO, Fe203, and SnO2, oxidize the pollutant in the presence of light having energy higher than the bandgap energy of the catalyst. When photons of sufficient energy levels fall on the surface of a catalyst, electrons and holes are created, and this initiates oxidation and reduction reactions over the catalyst surface.
During the photocatalytic oxidation process, light gets absorbed on the surface of the photocatalyst, which is generally a semiconductor having wide band gap, and this leads to the formation of electrons and holes on its surface. Owing to the continuous irradiation, absorption of light by the semiconductor catalyst occurs, and therefore the electrons move from the valence band to the conduction band forming positively charged holes and negatively charged elections in the valence and conduction bands, respectively. The presence of holes and electrons on the surface of photocatalyst gives rise to oxidation and reduction reactions.
The negatively charged electrons react with any positively charged pollutant molecules or react with electron acceptors such as O2 to generate the radical anion O2*. The positively charged holes generated by photocatalysis react with pollutant molecules and water molecules to produce OH- radicals, which subsequently oxidize the organic pollutant molecules.
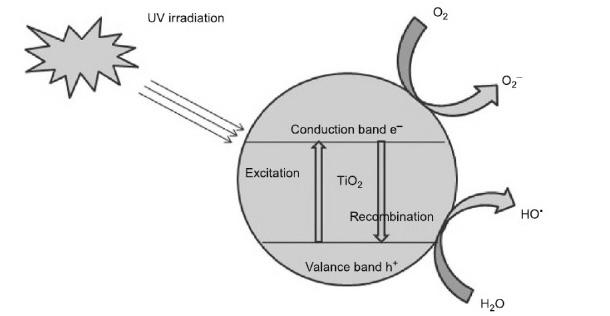
Schematic representation of photocatalytic process
M. Fenton Process
The concept of Fenton reaction was first suggested by Fenton, when he observed the oxidation of tartaric acid by H202 in the presence of ferrous (Fe2+) ions. This process is one of the prominent AOP that produces highly reactive OH for the degradation/mineralization of organic compounds. This process has been used as pretreatment or posttreatment for the degradation of pollutant from landfill leachate. During Fenton process, H202 reacts with ferrous ion Fe(Il) in acidic conditions to generate ferric ion, OH-, and hydroxyl anion.
These reactive species can oxidize organic and inorganic contaminants present in the solution. The reaction mechanism of the Fenton process is described. The ferric ion further gets reduced to ferrous ion and a peroxide radical by H202. The rate of ferric ion formation reaction of is higher than the rate of its reduction reaction, which proves that the production rate of Fe2+ ions is slower than its consumption rate. The OH- degrade organic compounds into CO2, H20, and various intermediate products. H202 also reacts with Fe3+ reducing to Fe2+
Moreover, many researchers have coupled Fenton process with other techniques such as photo-Fenton, sono-Fenton, and sono-photo-Fenton processes, thereby removing the pH barrier of Fenton process and enhancing the degradation rate of organic pollutants.
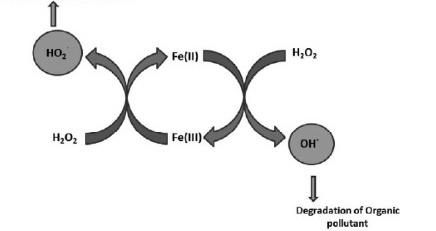
Schematic representation of Fenton process
Conclusion
The various wastewater treatment methodologies discussed in this chapter have their own merits and demerits regarding their application for wastewater treatment. The hybrid processes are proved to enhance the degradation rate owing to the increased production of OH- radicals. The demerits of photocatalysis and Fenton process such as noneffective distribution or scattering of UV light, nonuniform suspension of photo catalyst, settling of catalyst, regeneration, and lower production rate of OH- radicals can be overcome by combining photocatalysis and Fenton with ultrasonication and HC. One of the challenges to be explored include the utilization of solar (visible) light instead of UV source for photoexcitation of the semiconductor materials, which is also another aspect to be developed to reduce the cost of the hybrid process of photocatalysis and cavitation. As must of the research work has been on nanoparticles/photocatalytic materials synthesized using raw materials/chemicals available in the market, more focus should be on waste valorization in the synthesis of nano-photocatalysts, which will definitely reduce the overall cost of the process.
References
[1] https://www.mdpi.com/1996-1073/17/6/1400 [2] https://www.researchgate.net/publication/351410361_Advanced_technologies_for_wastewater_treatment_New_trends [3] https://www.sciencedirect.com/science/article/abs/pii/S221471442300096X [4] https://www.sciencedirect.com/science/article/abs/pii/S2215153221001926 [5] https://www.sciencedirect.com/science/article/pii/S2772416622000778#abs0002 [6] https://www.sciencedirect.com/science/article/abs/pii/S2214785321051129 [7] https://www.sciencedirect.com/science/article/abs/pii/S0045653523019604 [8] https://www.sciencedirect.com/science/article/abs/pii/S0043135424001143 [9] https://www.sciencedirect.com/science/article/abs/pii/S0043135423014975 [10] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4931528/ [11] https://www.sciencedirect.com/science/article/abs/pii/S004565352203017X [12] https://link.springer.com/article/10.1007/s40726-015-0015-z#:~:text=During%20the%20AOP%20treatment%20of,treatment%20prior%20to%20an%20ensuing [13] https://pubs.acs.org/doi/10.1021/acs.iecr.4c00794 [14] https://europepmc.org/article/med/36837685 [15] https://www.researchgate.net/publication/349313146_Advances_in_Wastewater_Treatment_I_Edited_by_Introduction_to_Conventional_Wastewater_Treatment_Technologies_Limitations_and_Recent_Advances
Copyright
Copyright © 2024 Spoorthy R, Sanjana Poojar , Pruthvi H R , Prakruthi YD. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63963
Publish Date : 2024-08-13
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

