Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Removal of Fluoride from Water by using Natural Adsorbent
Authors: Karuna C. Garud, Pratiksha P. Surse, Harshali E. Gunjal, Aditi S. Thorat, Devanand H. Kedare, Ravindra S. Sonavane
DOI Link: https://doi.org/10.22214/ijraset.2024.62808
Certificate: View Certificate
Abstract
Access to safe drinking water is not only the elementary need of human being but also honored as an mortal right .For overall development of the country , as acceptable nutrition, education, gender equivalency, and particularly the eradication of poverty in developing countries are required , along with that furnishing safe drinkable water is equally important . Fluoride one of the water quality parameters of concern, with redundant( beyond1.5 mg/ l, World Health Organization( WHO) guideline value of what contaminates groundwater coffers in numerous corridors of the world, and renders it not drinkable for mortal consumption, due to affiliated adverse health goods thus, knowledge of its junking, using the stylish fashion with optimum effectiveness demanded . Taking the inflexibility of the problem into consideration, the present paper aims to give a retrospective approach to the use of effective low- cost adsorbents for the junking of fluoride from water . The defluoridation capacity of certain low- cost natural adsorbents like Groundnut shell powder and Soya chunk powder were added in the list and discussed. The effect of pH, adsorbent dose, initial concentration , and contact time also delved . It\'s set up that the junking of Fluoride ions at optimum parameters like pH, adsorbent dose ,initial concentration and contact time is at 7, 2gm, 5mg/ l, and 100 minutes respectively for groundnut shell powder and 6, 2gm, 5mg/ l, and 100 minutes respectively for Soya chunk powder. For all optimum parameters, the maximum reduction in Fluoride ions observed in the range of 55 to 65% for groundnut shell powder whereas for soya chunk powder it\'s 40 to 50%
Introduction
I. INTRODUCTION
Water known as universal solvents because it dissolves most substance coming its contact. Safe drinking water is primarily need of everyone. Pure water is in limited quantity. Water may contaminated by naturally sources or industrial effluent. One such contaminant are fluoride.
Fluoride concentration in water recourse is increasing because of industrialisation and urbanisation. High concentration of fluoride in drinking water leads to various health effects like dental fluorosis and skeletal fluorosis .According the WHO standards, in drinking water, the fluoride should be within a range that slightly vary above and below 1 mg/L and in low water level, fluoride level is up to 1.5mg/L. According to Bureau of India standards, BIS (IS-10500), the desirable limit and allowable limit of fluoride in drinking water is 1.0 and 1.5 mg/L respectively. There are various methods for junking of fluoride from water like ion exchange , electrolysis , membrane separation and adsorption. But adsorption process is one of the best and most efficient method for junking of fluoride from water because of its initial cost , ease of operation , simplicity in design and flexibility. Hence, we used adsorption method junking fluoride testing. The efficiency of this techniques mainly depends on absorbents.
II. LITERATURE REVIEW
By golly, Palekar et al. done thought about takin' out fluoride using tea trash and drumstick as bio-adsorbents! When it comes to that fluoride stuff, teas ashy powder treated with acid was a smidge better than alkali-treated teas ashy powder at normal pH. But when it comes to MO or Moringa Oleifera, the alkali-treated MO powder done turned out better than the acid-treated MO powder at standard pH. Ya see, as the pH number goes up, the getting rid of that fluoride by sticking' it to these adsorbents goes up too, and it's at its peak at neutral pH. Both them their bio-adsorbents perform best when you throw in 400mg/lit of 'am and let 'end sit for 150mins, whether they're 212µ or 600µ. Them researchers reckon that the adsorption rate is quicker for the tiny 212µ particles because they got more surface area compared to them big of 600µ particles.
Ramesh et al., they totally checked out the batch column method for the deforestation of liquid solution using bottom ash. The period of contact with fluoride was recognized as 105 minutes at a 70mg/100ml bottom ash prescription. People received the maximal productivity of 73.5%.
To receive 83.2% of the selling efficiency, the pH was at 6. Similarly, the fluoride clearance tends to grow with a decrease in the atomic size. The most extreme monolayer absorption capacity of the bottom ash absorbent was clarified as 16.26 mg/g at 303 K. From the column method, it was recognized that the fluoride ions surge tends to grow in the bed height, because of an increase in the connection with time. Based on the Thomas model, the author viewed that the rate constant is 0.0619 l/min.mg, and the saturation point was recognized as 0.3714 mg/g. From the Yoon Nelson.
Saranya et al researched the DE fluoridization use bio adsorbents, like banana skin, enthusiasm fruit skid, furthermore enthusiasm fruit seed. Pre-eminent dismissal capability was accomplish by using banana skin powder. The writer terminated that the effectiveness of fluoride disposal ascends with increase in adsorbent dosage.
Aleena et al examined the defluorination of water by utilizing Moringa Oleifera and Tulsi. The author's observed that the removal of fluoride increases with an increased adsorbent dosing. The number of adsorbent doses were ranged from 0.1 mg/lit to 0.5 mg/lit in aqueous solutions. The authors also learned that for Moringa Oleifera bio-adsorbents, the maximal withdrawal capability of fluoride was 40% at 0.5 mg/lit while fluoride removal efficiency was 23% at 0.5 mg/lit by utilizing Tulsi as an adsorbent. Furthermore, the Moringa Oleifera seed flour was premier to Tulsi leaves for defluorination.
Sutapa Chakrabarty at al. explored the fluorine disappearance of tap water using neem stem as an adsorbent. The neem stem coal is perceived as an efficient adsorbent the fluoridation of drinking water origin. The adsorbent were effective with 94% efficiency in disposing of fluoride elements from watery solution of 10mg/l fluoride concentration. Biosorption balance was achieved inside 180 mins. The authors observed that the adsorption was dependent on pH and the most extreme adsorption achieved at pH of 5.0.
Bina Rani et al examined the defluoridation of water utilizing brick power as an adsorbent. The adsorbent efficiency of brick power was observed for fluoride removal from drinkable water samples of different concentrations. The writer inferred that adsorption of fluoride on brick power adsorbent from aqueous solution was considered as first order reaction and the mechanism of the removal of fluoride on adsorbent was found to be complex. The presence of other ions in groundwater didn't influence the defluorination procedure, in this way, showing that the brick power is an appropriate and economical adsorbent for fluoride.
III. METHODOLOGY
A. Preparation of Adsorbents
- Preparation of Groundnut Shell Adsorbent
Groundnut Shells were collected, have washed with tap water and dried in sun light for two days. Then dried shells dried again in the hot oven at 100°C for 24 hours. These groundnut shells get crush in mixer and sieved-. Then, the powder of groundnut shells being stored in some container for next use
2. Preparation of Soya chunk Adsorbent
Soya Chunks been collected and dry in sun for two days. This Soya Chunks be grind in a mixer and sieved. Then the powders of Soya Chunks be keep in a container for future utilization.
3. Preparation of Stock Solution
For equipped a regular sodium fluoride stock solution, we dissolve 0.2210gms dry sodium fluoride (NaF) in distilled water and diluted to 1000 ml(100 mg/l). Again, not diluted 100 ml of these stock solution to 1000 ml distilled water(10 mg/l).
1ml = 0.010 mg F.
B. Preparation of Reagents
- SPANDS solution
Dissolves 958 mg SPANDS [Sodium 2-( Parasulfophenylazo) -1,8- dihydroxy-3, 6 naphthalene desulphonated, also called 4,5-drihydroxy -3-(Parasulfophenylazo) -2,7 - napthalenedisulfonic acid tri sodium salt] in distil watered ant's dilute to 500 ml This solution is stables for at least 1 year if protecting from the direct sunlight.
2. Zirconyl-acid Reagents
Dissolved 133 mg zirconium chloride hexahydrate, ZrOC13.8H20, in approximately 25 mL distilled water Adding 350 mL concentrated HCl and diluting to 500 milliliters with distilled water.
3. Acid Zirconyl-SPADNS Reagent
Mix equalled size of SPADNS solutions and zirconyl-acid reagent. The combined reagent are stabled for almost 2 years.
Spectrophotometer providing light path of 1 cm at 570nm was utilized for analysis.
4. Preparation of standard calibration curve
Fluoride standards were prepared within the range of 0 to 1.40 mg/l through diluting quantities of standard fluoride solution in 50 ml distilled water. Pipette 5.00 ml each of SPANDE’S solution and zirconium acid reagent, or 10.00 ml mixed acid-zirconium-SPANDES reagent, to each standard and mix goodly. Contamination should be not be avoided. Spectrophotometer is to be set to zero absorbance with the reference solution and absorbance readings of standards obtained. An encompassing curve of the milligram’s fluoride-absorbance relationship was plotted.
C. Tests on Adsorbent
- pH
The pH scale is logarithmic, corresponding to the negative of the base 10 logarithm of the molar concentration (measured in moles per litre) of hydrogen ions in a solution. More exactly, it is the negative of the base 10 logarithm of the hydrogen ion's activity. At 25 °C, acidic solutions have a pH less than 7, while basic solutions have a pH greater than 7. The pH's neutral value is temperature dependent, falling below 7 as the temperature rises. The pH might be less than zero for very strong acids and greater than 14 for very strong bases.
- Procedure
- Add 10gm of sample to 100ml of distilled water.
- Transfer the sample to a beaker and shake it for 15 minutes at 80 rpm.
- After holding the sample for 15 minutes, the solution is filtered using Whatman paper.
- Use a digital pH metre to test the pH of the resulting filtrate.
- Results:
- pH of soya chunk powder - 6
- pH of groundnut shell powder – 5
2. Electrical conductivity
Electrical conductivity is a measurement of a material's capacity to carry electrical current. Electrical conductivity is also called specific conductance.
- Procedure
- Add 10 gramme of sample to 100 ml of distilled water.
- Transfer the sample to a beaker and shake it for 15 minutes at 80 rpm.
- After holding the sample for 15 minutes, the solution is filtered using Whatman paper.
- Use an electrical conductivity meter to test the resulting filtrate.
- Results:
- Conductivity of soya chunk powder – 5795μS
- Conductivity of groundnut shell powder – 2490μS
3. Specific gravity
Specific gravity is the ratio of a material's density to that of a reference substance; in other words, it is the ratio of a substance's mass to that of a reference substance for the same volume. The apparent specific gravity of a material is the weight of that volume divided by the weight of an equal volume of the reference substance. The reference substance for liquids is almost always water at its densest (4°C or 39.2°F); for gases, it is air at room temperature (20°C or 68°F). Nonetheless, both the sample and the reference must have their temperatures and pressures indicated. Pressure is almost always one atm.


Conclusion
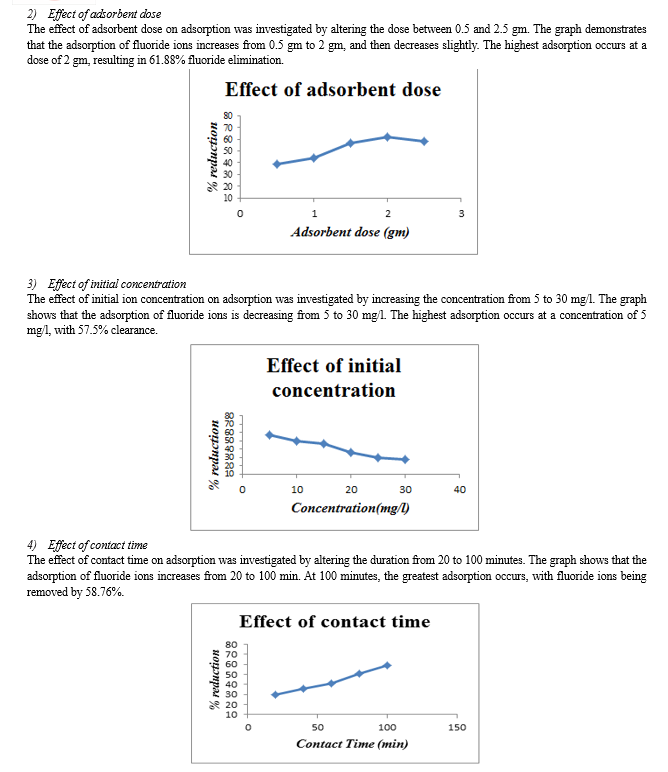
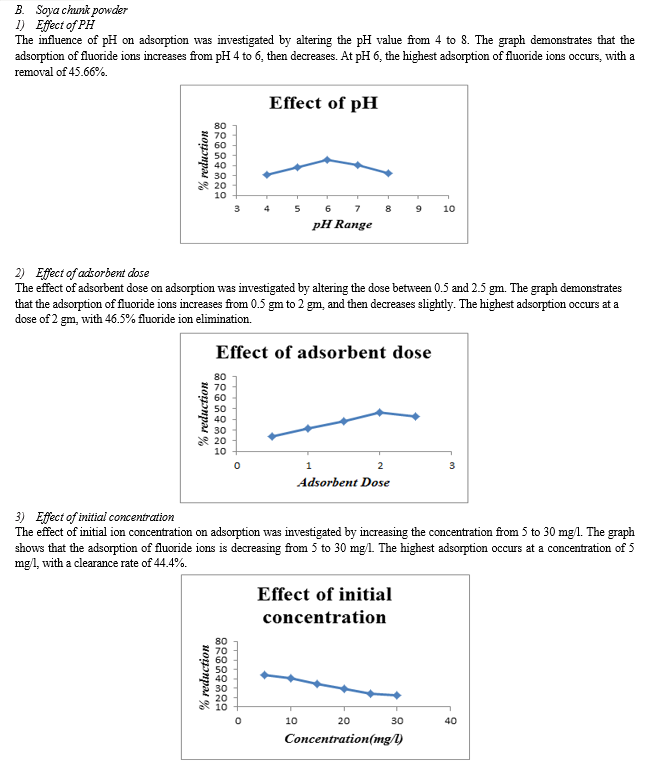
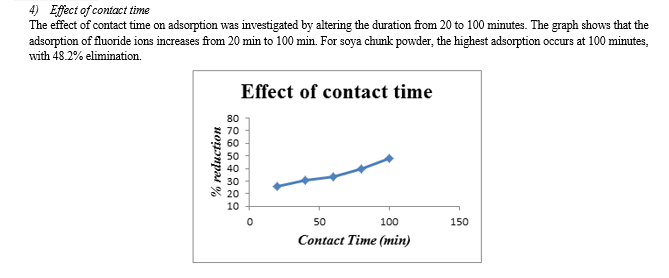
1) Groundnut shell powder reduces fluoride ions by 55% to 65% for all optimal values, while soya chunk powder reduces them by 40% to 50%. 2) Soya chunk and groundnut shell have the highest percentage of fluoride ion elimination at pH 6 and 7, respectively. 3) Increasing adsorbent dosage led to higher % removal, with a maximum of 2 g for groundnut shell and soya chunk. 4) Adsorption decreased with increasing initial fluoride ion concentration. Both adsorbents achieve their highest percentage removal at an initial concentration of 5mg/l. 5) For both adsorbents, the maximum percentage removal occurs after 100 minutes of contact time.
References
[1] A.S. Parlikar, S.S. Mokashi, \" A Comparative Study of Defluorination of Water by Tea Waste and Drumstick as Bio-adsorbents\", International Journal of Multidisciplinary Research and Development, Vol, 2, July 2015 [2] T. Ramesh, R. Gandhimathi, P. V. Nidheesh and M. Tay wade,” Batch and column operation for the removal of fluoride from aqueous solution using bottom ash’’ Environmental Research, Engineering and Management 2012 [3] S. R. Saranya and N. Anu, “ Comparative Study of Fluoride Removal from Synthetic Wastewater by using Bio-adsorbents”, International Journal of Scientific & Engineering Research, Volume 7, Issue 4, April-2016 [4] Aleena R Haneef and Nithya Kurup, “ Comparative Study of FluorideRemoval from Water by Using Moringa Oleifera and Tulsi (OcimumSanctum)”, International Journal of Scientific & Engineering Research,Volume 7, Issue 4, April-2016 [5] Sutapa Chakrabarty, H.P.Sarma, “Defluoridation of contaminated Drinking water using neem charcoal adsorbent:Kinetics and equilibrium studies”, International Journal of Chem Tech Research, April-June 2012 [6] Bina Rani, Raaz Maheshwari, Chauhan, A. K., Bhaskar, N. S, “Defluoridation of contaminated water employing brick Powder as an adsorbent”, International Journal of Science and Nature, vol. 3(1) 2012: 78-82 [7] Renu Singh, Shiv Pratap Raghuvanshi and C.P. Kaushik, “Defluoridation of Drinking Water using Brick Powder as an Adsorbent”, Asian Journal of Chemistry, Vol. 20, No. 8 (2019) [8] Vinish V Nair1, Anandu Aravind2, Dayal Kurian Varghese,” Defluoridation of water by composite bed of low-cost bio-adsorbents”, International Journal of Advanced Technology in Engineering and Science,Vol.no.4, Issue no. 2, February 2016 [9] G.R.Kiran Kumar, M. Shambavi Kamath and Praveen S Mallapur, “Defluoridation of Water by using Low Cost Activated Carbon Prepared from Lemon Peels”, Journal of Basic and Applied Engineering Research, Volume 3, Issue 8; April-June, 2016, pp. 658-660 [10] Dr . D. N. V. Satyanarayana, Smt. M. Sudheera, “ Removal of fluoride Using Brick Powder by Adsorption”, International Journal of Research and Analytical Reviews, volume 2, issue 4,Oct. – Dec. 2015.
Copyright
Copyright © 2024 Karuna C. Garud, Pratiksha P. Surse, Harshali E. Gunjal, Aditi S. Thorat, Devanand H. Kedare, Ravindra S. Sonavane. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET62808
Publish Date : 2024-05-27
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

