Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
A Comprehensive Review of Current Immunotherapies and Their Therapeutic Applications in Cancer Treatment
Authors: Arhaan Kohli
DOI Link: https://doi.org/10.22214/ijraset.2024.64285
Certificate: View Certificate
Abstract
This paper presents a literature review on current advancements in cancer immunotherapies. It begins by examining the current cancer epidemiology, highlighting the prevalence and impact of various cancer types. The paper then explores the hallmarks of cancer, focusing on the biological capabilities that enable tumor growth and survival. Following this, the tumor immune microenvironment is discussed, emphasizing its role in tumor progression and immune evasion. The concept of cancer immunoediting is introduced, outlining the dynamic interactions between the immune system and tumor cells. The review then delves into specific immunotherapeutic approaches, including monoclonal antibody therapy, immune checkpoint inhibitors, CAR T-cell therapy, and cancer vaccines. Each therapy is analyzed in terms of its mechanism of action, clinical efficacy, and current applications. The paper concludes with a discussion on the challenges, limitations, and future directions of cancer immunotherapies, providing insights into how these treatments may evolve to improve patient outcomes.
Introduction
I. INTRODUCTION
A. Incidence Of Cancer
Cancer is a disease in which some of the body’s cells grow uncontrollably and spread to other parts of the body. Fundamentally, cancer is a disease of the genome which arises from the accumulation of mutations over time. Uncontrolled proliferation by transformed cells (subject to evolution by natural selection) may constitute lumps or aggregations of tissue known as tumors (Brown et al. 2023). Overall, cancer poses the highest clinal, social, and economic burden in terms of cause-specific Disability-Adjusted Life Years (DALYs) among all human diseases. Across ages 0-74, the risk of developing cancer in humans is approximately 20.2% (Mattiuzzi & Lippi, 2019).
This risk percentage varies slightly among males and females with a distribution of 22.4% in males and 18.2% in women (Mattiuzzi & Lippi, 2019). In 2018, of a total of 18 million new cases were observed.
The most prominent cancers observed were lung cancers (2.09 million cases), breast cancers (2.09 million cases) and prostate cancers (1.28 million cases). With regards to mortality, cancer is the second worldwide cause of death (8.97 million deaths), with lung, liver and stomach cancers being the deadliest. A more recent study, by the International Agency for Cancer Research (IARC), showed close to 20 million new cases of cancer and 9.7 million deaths in the year 2022, with a distribution of cancers very similar to the ones observed in 2018.
Incidence rates of cancer varied four-fold to five-fold across world regions (figure 1). The Epidemiology of Cancer in 2022 was similar to those observed in previous years. Asia was the largest area of incidence, totaling out to 49.2% of all cancer cases in 2022, China being the largest contributing country. The Americas constitute 21.1% of new cases, 22.4% of new cases in Europe and 1.4% in Oceania. These statistics are influenced by a variety of factors, including but not limited to the availability of screening facilities, population and Human Development Index (HDI) (Bray et al. 2022).
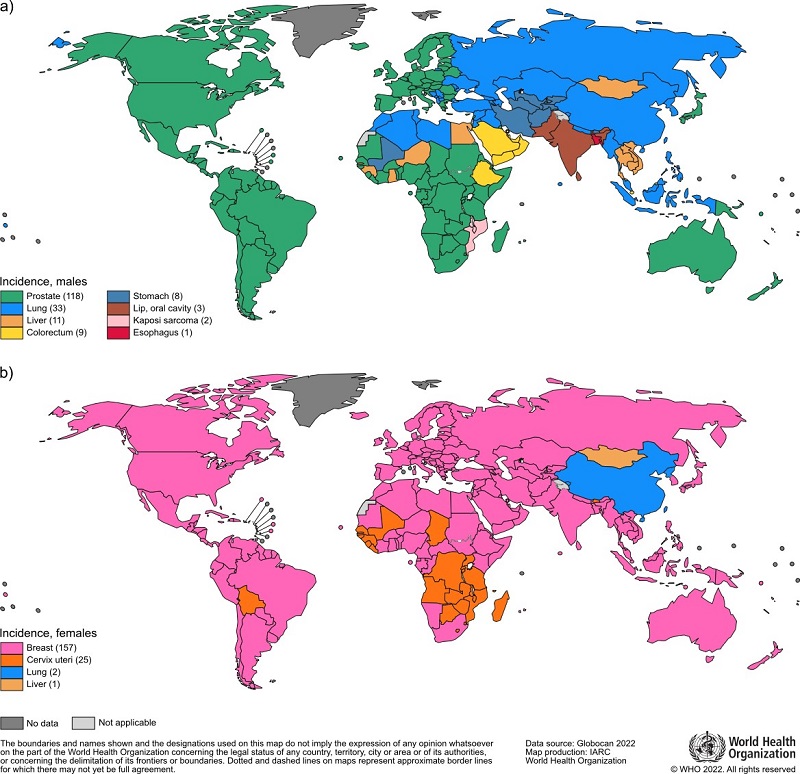
Figure 1. Global incidence of cancer in males and females. Adapted from WHO, 2022 (United Nations Procurement Division/United Nations Development Program)
As is commonly known, cures or treatments for cancers present to be highly intense, with a plethora of side effects observed in treatments such as chemotherapy, surgery and radiation therapy. Surgeries tend to be invasive, and even with these treatments, complete removal of the cancer is not guaranteed. Cancer survivorship and treatment depend on several factors including stage of cancer, type of cancer and access to care. Testicular cancer has a global survivorship rate of 98%, while pancreatic cancer is several times more deadly with a survival rate of roughly 1%. Many of the most commonly diagnosed cancers have a ten-year survival rate of 50%. Less than 20% of people whose cancers were difficult to diagnose and treat survive their cancer for ten years or more (Cancer Research UK, 2023). With the above being said, it is evident that there is no current remedy or cure for cancer. The lack of a viable treatment option makes immunotherapy extremely promising in the field of cancer treatment. Harnessing the strengths of our immune system, immunotherapy works by boosting or strengthening existing lymphocytes, T-cells, Natural Killer (NK cells) and dendritic cells (to name a few), making them increasingly sensitive to abnormalities in the body. Research in the area is crucial, and propels us one step forward towards finding a cure for cancer.
This research paper will discuss the methods, mechanisms and pathways to harness immunotherapy in cancer treatment. The paper explains the hallmarks of cancer, providing a detailed description of the disease. The tumor microenvironment discusses the different cells present in the cancerous area and their roles in the cancer cycle. Immuno-oncology forms a major part of the paper, it explores immunotherapy as a possible solution for cancer treatment. The discussion integrates the findings in the above mentioned sections to give a well structured review on immunotherapy as a possible treatment for cancer.
B. Hallmarks Of Cancer
Cancer is often categorized by several biological pathways, changes and capabilities acquired during the multistep development of human tumors. In a broad sense, tumors can be of two types- benign or malignant. Benign cancers refer to growths confined to a specific site, without invasion of adjacent tissues. The epithelial growth has not yet penetrated through the basement membrane, allowing relatively easy treatments. Malignant aggregations on the other hand become invasive. The tumor environment is not well differentiated, and cells grow and divide rapidly. These cancers are typically harder to treat.
Apart from this classification, cancer can be split into four main types: carcinoma (cancers affecting epithelial tissues in the internal and external linings), Sarcoma (Cancer of connective tissues found in bones, tendons, muscles and fat), Leukemia (cancer of the blood) and Lymphoma (cancers of the lymphatic system). Despite these different classifications, cancers typically stem from similar sources, with genetic mutations being the most common.
Genetic mutations come from a variety of places including exposure to radiation, chemical imbalances, exposure to toxins (such as those caused by tobacco), chronic inflammation and genetic predispositions. All these exposures tend to cause genetic mutations, which deregulates homeostatic biological function. In some instances, mutations accelerate certain pathways, such as in the activation of protooncogenes. For example, the Sarcoma (SRC) oncogene activation, the Epidermal Growth Factor Receptor (EGFR) oncogene activation and the Phosphoinositide 3-Kinase (PI3K) pathway (Pelaz & Tabernero, 2022).
Cancer is typically identified by characteristic features, referred to as ‘hallmarks.’ The hallmarks of cancer are divided into ten segments, each describing a commonality found in all cancers as proposed by Hanahan and Weinberg in 2011. These hallmarks are briefly discussed in the following paragraphs to give an adequate understanding on the survival of cancer, metastasis of cancer and the mechanisms by which it thrives in the body. The figure provided (figure 2.) diagrammatically represents these hallmarks under their headings and provides a possible solution to overcome the effects of each hallmark.
1) Sustained Proliferative Signaling
Cancers have the ability to sustain chronic proliferation. Cancerous cells, as opposed to normal cells, do not require external signals to divide, as oncogenes drive proliferation. This process of sustained proliferative signaling can be achieved in a variety of ways. A common example includes the overexpression of growth factor receptors. In a normal scenario, Epidermal Growth Factor Receptor (EGFR), a receptor tyrosine kinase, binds to ligands. This allows the dimerisation process to initiate. The homodimers formed become auto phosphorylated which activate the EGFR pathway, allowing the cell to divide (Oda et al., 2005). When mutated in a cancerous case, point mutations (for example valine to glutamine) allow constant autophosphorylation. This leads to intrinsic activation and rapid proliferation, allowing tumors to form. When EGFR is activated in cancer cells, it affects downstream signaling routes such as the Ras/ Raf/ MEK/ ERK pathway which again lead to cell division (Uribe et al. 2021) Similarly, the PI3K pathway can also drive proliferation, a mutation in the E17 gene can allow for AKT to be synthesized rapidly, allowing PI3K to stay in an active form, driving signaling for rapid cell division (Khezri et al. 2022)
2) Evading Growth Suppressor and Resisting Cell Death
Cancers tend to evade normality and continue proliferation by ‘driving off’ suppressors. Common tumor suppressor genes include Retinoblastoma (RB) protein and the P53 protein. pRB acts as a gatekeeper, integrating signals to decide whether the cell cycle should continue. A mutation in the pRB gene (particularly RB1) can lead to inactive pRB proteins. Alternatively, overexpression of cyclins and cyclin-dependent kinases (CDK’s) can phosphorylate and inactivate pRB, stopping pRB from ‘arresting’ the cancer's rapid growth (Chinnam and Goodman, 2013)
P53 is known as the ‘guardian of the genome’ because it induces apoptosis, killing off cells that divide irregularly, arresting uncontrolled proliferation upon identification of genomic mutations. The TP53 gene governs the apoptosis program, a mutation in this gene would render p53 ineffective because p53 functions as a tetramer. Alternatively, cancer will try to downregulate tumor suppressor genes by overexpression of their negative regulators for example Mouse Double Minute 2 (MDM2) (Hu et al. 2021). As a negative regulator, it downregulates the function of p53 thereby preventing p53 induced apoptosis in cancer cells (Hu et al. 2021)
3) Enabling Replicative Immortality
In relation to this, the next hallmark discusses ‘Enabling Replicative Immortality’. Multiply sources of evidence point that telomeres protect the ends of chromosomes, increasing the capacity for unlimited proliferation.
In normal cells, each successive division results in a small portion of telomeric DNA getting lost, leading to progressive telomere shortening, making the chromosomes more vulnerable to damage. When the telomeres reach a critically short length, a permanent arrest stage known as senescence occurs. Cells that bypass this arrest go on to die in apoptosis. In cancer cells, telomere maintenance mechanisms are activated in a variety of ways. Mutations cause activations of telomerase, which counters telomere shortening. At the same time, cancer cells utilize other mechanisms known as Alternative Lengthening of Telomeres (ALT) to achieve the same lengthening of telomeres (Dilley & Greenberg, 2015). The new longer and unharmed telomeres protect the DNA sequence, making the cell attain replicative immortality.
4) Angiogenesis and Activation of Invasion and Metastasis
Angiogenesis, the formation of new blood vessels from pre-existing ones, is crucial in the tumor microenvironment (TME) as it supplies the growing tumor with nutrients and oxygen, which are critical for its continued growth and survival. Without angiogenesis, the tumor would be unable to expand beyond a certain size due to limited access to these resources. New blood vessels ensure that the tumor receives adequate blood flow, facilitating sustained growth. Additionally, efficient blood flow via new blood vessels helps in the removal of metabolic waste products from the tumor, preventing a toxic microenvironment that could inhibit tumor growth.
Angiogenesis also plays a vital role in metastasis, providing pathways for cancer cells to enter the bloodstream and spread to distant organs. This process is a major contributor to cancer progression and the primary cause of cancer-related mortality. Furthermore, tumors often experience hypoxic conditions (low oxygen levels) due to rapid cell division. Angiogenesis helps to alleviate hypoxia by increasing oxygen delivery, which can further drive tumor aggressiveness and resistance to therapy. Fibrins from fibroblasts carried by newly formed blood vessels help in providing structural support to the tumor, as well as strengthening the tumor blood supply(Liu et al., 2023).
Not long after, invasion and metastasis are activated which allows cancer cells to invade into surrounding tissues and escape into secondary organs (only if the basement membrane has been penetrated). Cancer downregulates or mutates E-cadherin, a protein critical for cell-cell adhesion in epithelial tissues. This loss reduces cell adhesion allowing cancer cells to detach from neighboring cells and invade surrounding tissues. Simultaneously, Epithelial-Mesenchymal Transition (EMT) occurs where epithelial cells lose their polarity, acquiring mesenchymal characteristics that promote motility and invasion. The induced angiogenesis may aid in the cell’s movement (Ribatti et al. 2020).
5) Deregulating Cellular Energetics
Cancer cells rewire their metabolism to focus on growth, survival and long-term maintenance. The Warburg effect begins to take place in a hallmark known as ‘Deregulating Cellular Energetics’ (Liberti and Locasale, 2016). There is an increased glucose uptake within cancerous cells. Cancer cells preferentially undergo glycolysis even in the presence of oxygen. This frees up more phosphate molecules allowing ATP to be synthesized needed for rapid proliferation. Simultaneously, mitochondrial dysfunction begins to occur. Cancer cells downregulate oxidative phosphorylation (carried out by the mitochondria) partially due to mutations in genes encoding mitochondrial proteins or regulatory pathways that control mitochondrial function.
6) Evading Immune Destruction, Genome Instability and Promotion of Inflammation
The final three hallmarks focus on evading immune destruction, genome instability and promotion of inflammation. Tumor cells evade the immune system in a complex process known as immune editing. Immune editing in cancer encompasses three phases: escape (avoiding immune detection), equilibrium (resisting immune attack), and editing (adapting to immune pressure). The mechanisms and pathways behind these steps are discussed in great detail in the next sections. Genome Instability implies a high frequency of DNA mutations caused by defects in processes of how DNA is repaired and replicated. It is these mutations that drive all the changes that cancer establishes through the tumor. Finally, cancer is known to promote inflammation. As part of the immune editing, cancer cells hijack inflammatory mechanisms to promote their own growth and survival. Anti-tumor immune cells are subverted into tumor-promoting immune cells that secrete pro-survival and pro-migration factors that allow tumor growth and metastasis.
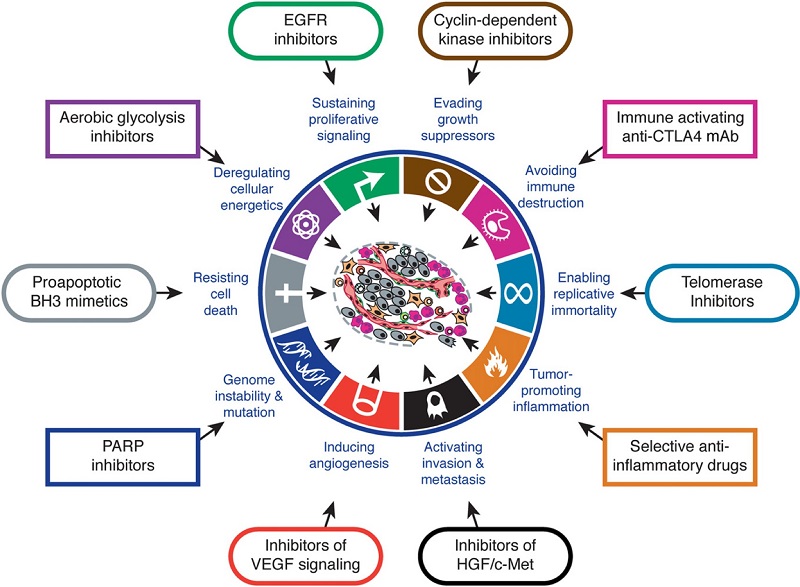
Figure 2. Hallmarks of cancer with certain ‘treatments’ for each hallmark. Adapted from Hannahan and Weinberg, 2011, The Hallmarks of Cancer (Hanahan & Weinberg, 2011)
II. THE TUMOR-IMMUNE MICROENVIRONMENT
The tumor microenvironment (TME) refers to the complex environment surrounding a cancerous tumor, consisting of various cellular and non-cellular components. The TME includes interactions between cancerous cells, immune cells and proteins which together play a crucial role in tumor development, progression, and response to therapy. The TME is majorly composed of cancer cells and bone-marrow derived cells (BMDC’s), which include Macrophages, Mesenchymal Stem Cells (MSD) and Myeloid-derived suppressor cells (MDSC’s), among many others (Anderson & Simon, 2021). The TME is also known to have a rich blood supply, as is discussed under the hallmark of cancer ‘Angiogenesis’ (Hannahan & Weinberg, 2011). Angiogenesis gives rise to new blood vessels which feed the tumor microenvironment, allowing signaling molecules, cells and proteins to reach the tumor site. The blood supply also aids the cancer from metastasizing further.
A prominent activity of the Tumor-immune microenvironment is the epithelial to mesenchymal transition (EMT). The EMT involves the transition of epithelial cells into mesenchymal phenotypes which promote tumor progression and metastatic expansion, thereby allowing the progression of cancer. The EMT process is characterized by changes in cell-cell adhesion, changes in the polarity of certain cells, remodeling of the cytoskeleton, and changes in cell–matrix adhesion, all of which result in improvement of migratory and invasive properties in cancerous cells (Roche, 2018). EMT inducers include specific cytokines and growth-factors secreted within the tumor-immune microenvironment. Concluding the above, the EMT acts as a method to make cancer cells more invasive and increasingly metastatic.
Homeostasis in normal tissues requires a balance of cell proliferation and death which is maintained through intercellular communication. The extracellular matrix (ECM) plays a crucial role in regulating this communication. The ECM acts as a physical scaffold facilitating interactions between different cells. Certain adhesion molecules such as β1 integrins and epithelial (E)-cadherin help this scaffold stay together, preventing the uncontrolled proliferation of a group of cells. Comparing the above to a cancerous case, it is observed that mutations lead to ECM stiffening- a process in which cancer-associated fibroblasts (CAFs) and other stromal cells often produce excessive amounts of ECM components, such as collagen, fibronectin, and laminin (Goodman, 2020). This leads to ECM stiffening which promotes cancer cell proliferation and invasion. Alongside this, Matrix metalloproteinases (MMPs) (among other proteinases) are often upregulated in cancer. This leads to the degradation and remodeling of ECM components creating pathways for cancer cell metastasis and invasion. ECM stiffening can also influence gene transcription via mechanotransduction (the process by which cells convert mechanical signals from the environment into biochemical signals).
This involves sensing ECM stiffness through integrins, transmitting forces through the cytoskeleton and activating signaling pathways, ultimately leading to changes in gene expression and cellular behavior (Selig et al. 2022).
The following figure (figure 3) depicts the tumor microenvironment.
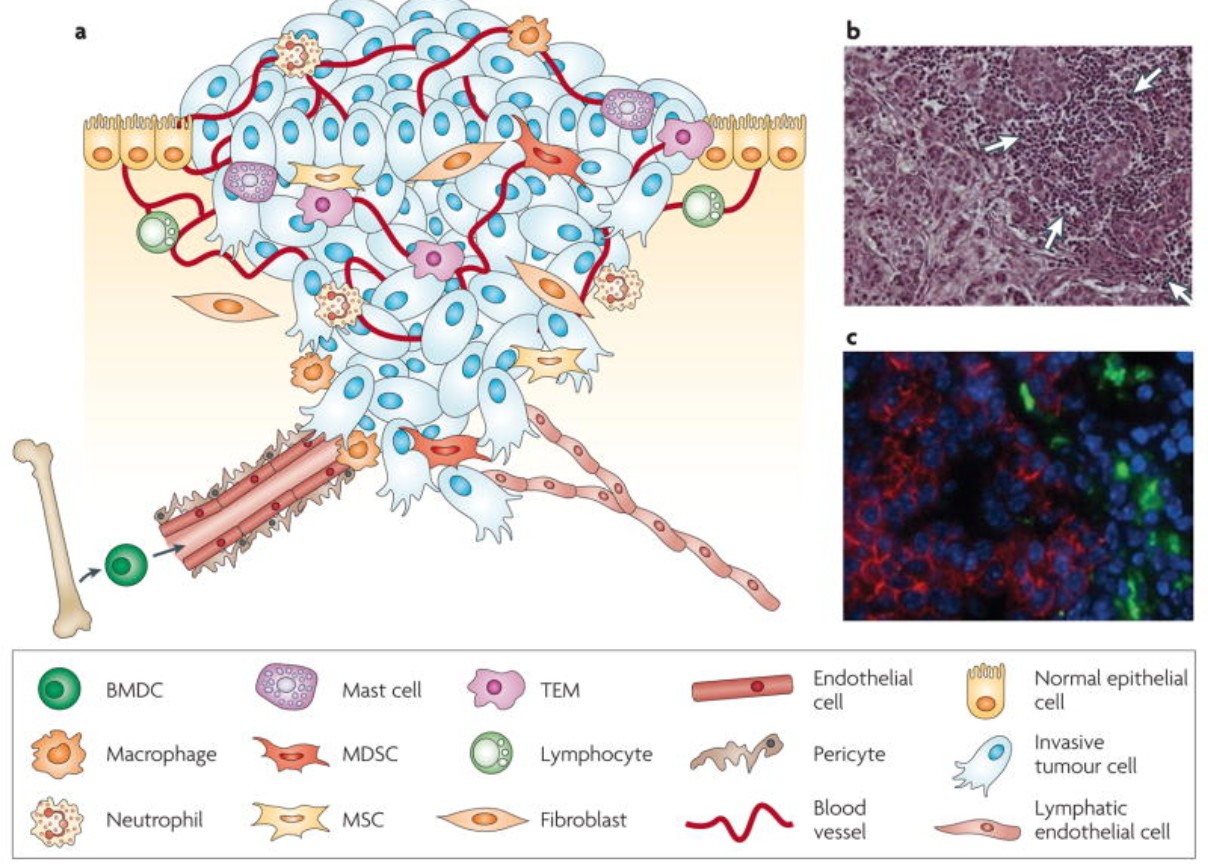
Figure 3. The Tumor Microenvironment. Adapted from Johanna A. Joyce and Jeffrey W. Pollard, 2009, Microenvironmental Regulation of Metastasis (Joyce & Pollard, 2009)
Bone Marrow Derived Cells (BMDC) in The Tumor Microenvironment (TME)
The presence of leukocytes in the TME was generally thought to be a consequence of a failed attempt at cancer cell destruction. This, however, was not fully true as shown by Joyce and Pollard, 2009. The interaction between tumors and the immune system, however, is far more complex.
Tumors have the ability to render certain inflammatory cells tumor promoting, rather than tumor suppressing. This process of cancer immunoediting is more apparent in cancers associated with chronic inflammation, where the initial inflammatory response is not resolved, and systemic conditions promote the continued recruitment of BMDC to the tumor site, making the cancer metastasise and develop faster. A chronic inflammatory state can set up a cascade of events with tumor-promoting consequences, rendering the BMDC cells as a danger, rather than an aid.
Macrophages have been shown capable of modifying cancer behavior and have been shown to promote angiogenesis, invasion, intravasation and metastasis in animal models. Inherently, macrophages are considered plastic cells due to the high adaptability. (Joyce & Pollard, 2009) Cancers harness this very adaptability to exploit the macrophage’s capabilities, allowing it to promote proliferation. Tumor associated macrophages (TAM) use the paracrine loop in which EGF produced by TAMs increase the invasiveness and migration to neighboring breast cells that express the EGF receptor (EGFR). Cancer cells in turn express CSF-1, which acts as a potent chemoattractant for other CSF1R (CSF1 receptor) expressing macrophages. This positive feedback loop allows the presence of TAM to every site of cancer metastasis, allowing proliferation to be quicker and easier (Joyce & Pollard, 2009).
Other examples of BMDC include myeloid-derived suppressor cells (MDSC) which suppress the adaptive immune response by blocking specialized T-cells. MDSCs produce interleukin 10 (IL-10) which, when overexpressed, achieves the same (Gabrilovich and Nagaraj, 2010). Dendritic cells inhibit the immune response and promote a tolerogenic environment, and mesenchymal stem cells (MSC) are shown to aid in dissemination of cancer cells.
In conclusion, the TME hosts a variety of cells, each adapted to aid the cancer in a particular way. Epithelial cells are converted into mesenchymal phenotypes, as is discussed in the EMT section. Macrophages modify cancer behavior and promote angiogenesis. Other BMDCs such as MDSCs block T cells, while MSCs aid in the metastasis and spread of cancer. Together, the various gene-edited cells of the TME play a crucial role in the tumor's survival, maintenance, immune escape and proliferation.
III. CANCER AND THE IMMUNE RESPONSE- IMMUNOEDITING
Cancer arises from genetic errors that escape DNA repair mechanisms leading to disrupted pathways, uncontrolled cell proliferation, ineffective tumor suppressor proteins (p53, pRB), new blood vessel formation, failed apoptosis, and resource program depletion. Despite all of these changes, cancer remains notoriously hard to flag and not only evades the immune system but also harnesses it for its own benefit. This section explores the vast mechanisms cancer possesses to harness the immune system and use it as a tool for its own metastasis.
Paul Elrich first gave a plausible concept for immunological surveillance in which he stated ‘‘I am convinced that during development and growth malignant cells arise extremely frequently, but in the majority of people they remain latent due to the protective action of the host. I am also convinced that this natural immunity is not due to the presence of antimicrobial bodies but is determined purely by cellular factors. These may be weakened in older age groups where cancer is more prevalent.’’ This initial theory is developed today and forms the basis for initial cancer detection by our immune system. The immune response to cancer initially develops in a relatively straightforward way.
Mutations in the genome of cancerous cells code for different proteins and thus, different amino-acids. If these abnormal proteins are expressed on the cell surface, they are known as neo-antigens and are perceived as ‘non-self’ by the immune system. The Major Histocompatibility Complex (MHC Complex) plays a crucial role in helping the immune system identify these abnormalities. A fundamental process of the immune system, MHC presents certain molecules on the surface of all nucleated cells which allow T-cells to identify it as ‘self’ or ‘non-self’ (Guler, 2018). MHC I occurs in all nucleated cells while MHC II occurs in antigen-presenting cells (APC) only (Swain & McKinstry, 2016). Neoantigen peptides are loaded onto MHC molecules within the endoplasmic reticulum, where they are subsequently transported to the cell surface for presentation. This allows the cancerous cell to be flagged as ‘abnormal’ by T-cells (specifically CD8+ cytotoxic T cells and CD4+ helper T cells). Following detection, the effector phase initiates in which cancer cells are eliminated. Activated T cells release cytotoxic granules containing perforin and granzymes which induce apoptosis in cancer cells (Janeway, 1970). Cytokines such as interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) which further contribute to tumor cell destruction. Despite this mechanism being present, cancer cells possess the ability to immuno-edit. Cancer immunoediting is the process where the immune system recognizes and eliminates cancerous cells (elimination), keeps them in check (equilibrium), or fails to control their growth leading to tumor progression (escape). The three stages are discussed in detail below.
A. Elimination Phase
As discussed above, the immune system first flags the cancer cells and proceeds to eliminate them using the MHC complex.
B. Equilibrium Phase
Some cancer cells may survive the initial immune attack by different mechanisms. For example, the cancerous cells may downregulate the MHC I molecules or disrupt MHC I machinery on their surface making detection difficult. Simultaneously, expression of immune checkpoint molecules (due to mutations) such as programmed death-ligand one (PD-L1) binds to T-cell receptors, leading to exhaustion and dampening of the immune response. Induction of immunosuppressive factors and resistance to apoptosis (due to mutations) may make the cancerous cells even more resistant to immune-elimination (Cornel et al. 2020)
This would allow the cancerous cells to reach a state of equilibrium and dormancy. In this stage, growth of the cancer is extremely limited. It is regulated by immune surveillance and continues to develop at a slow rate, hiding from immune detection and destruction. The immune system exerts selective pressure on tumor cells, favoring the outgrowth of less immunogenic variants.
C. Escape Phase
Cancer cells that acquire genetic mutations or epigenetic changes can evade immune recognition and destruction. This will allow uncontrolled proliferation of cancer cells and will mark the onset of a resistant and hard-to-treat cancer.
There is an upregulation of immune checkpoint molecules. Molecules such as PD-L1 are upregulated by cancer cells. The PD-L1 binds to PD receptors on T-cells, this signaling pathway leads to T cell exhaustion and deactivation.
This accelerated pathway now paves the way for uncontrolled proliferation, as T-cell function is hindered (Mittal et al. 2014). Other immune checkpoint molecules with similar functions include cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). Following this, there occurs an induction of immunosuppressive cells. Cancer cells and the tumor microenvironment recruit regulatory T-cells known as Tregs. These cells suppress the activation of effector T cells and dendritic cells while also upregulating immunosuppressive cytokines such as interleukin 10 (IL-10) and transforming growth factor-beta (TGF-β). This process hinders the immune system, again paving the way for uncontrolled proliferation. MDSCs are another population of immunosuppressive cells recruited by tumors. They develop in a similar way to Tregs and release similar immunosuppressive cytokines. Among all of these changes, cancerous cells continue to downregulate MHC I molecules and destroy/ alter MHC machinery to avoid detection by immune cells. It is similar to the MHC disruption in the equilibrium phase but on a larger scale (O’Donnell et al. 2018)
The diagram below (figure 4.) sums up the mentioned stages and additionally represents the downregulation and upregulation of certain proteins critical to cancer immunoediting.
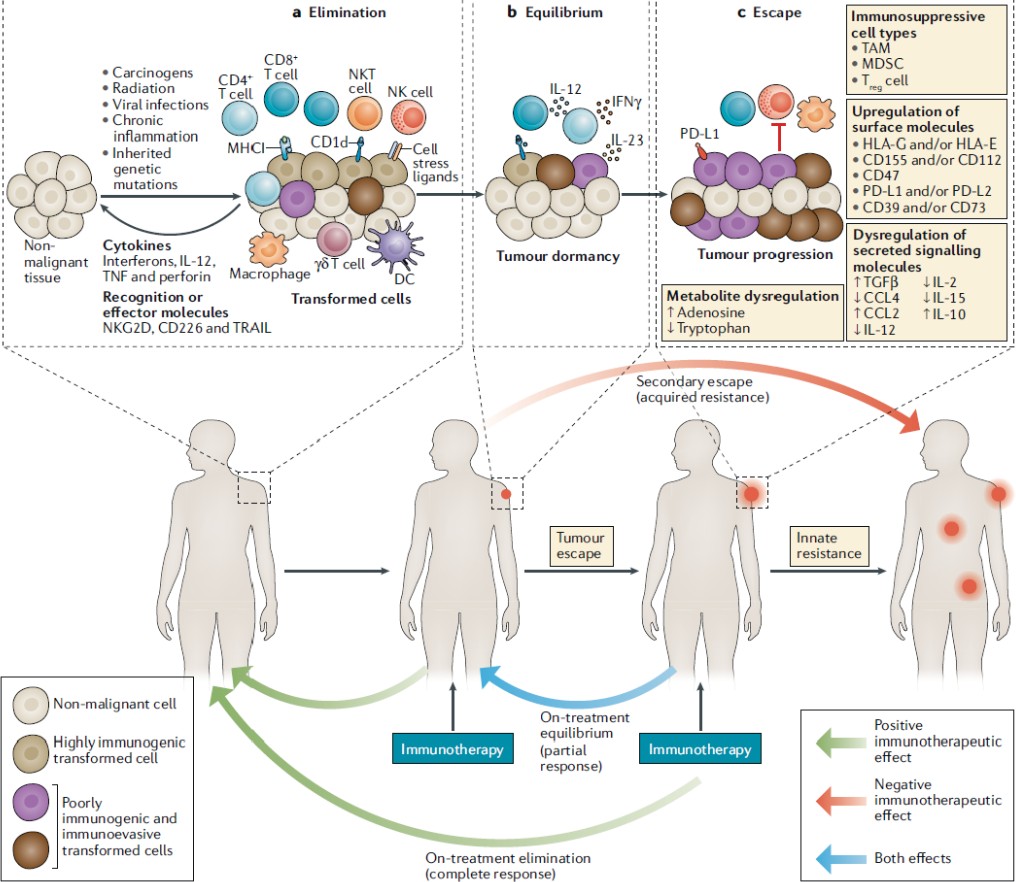
Figure 4. The Different Stages of Cancer Immunoediting. Adapted from Jake S. O’Donnell, Micheal W. L. Teng and MarkJ. Smyth, 2018, Cancer immunoediting and resistance to T cell-based immunotherapy (O’Donnell et al. 2018)
Tumors and their interaction with the immune system can be broadly classified into two types: Hot or cold. Each type of tumor comes with its own characteristics. However, it is noticed that hot tumors present a better prognosis and are easier to treat with approaches such as immunotherapy. That is precisely why there is a race to make cold tumors hot.
Hot tumors, also known as inflamed tumors show a high level of immune cell activation. There is significant inflammation at the sight with a relatively large presence of T-cells. These tumors often tend to express immune checkpoint molecules like PD-L1 which serve to exhaust and deactivate T-cells. The nature of this tumor, paired with the high immune activity make immunotherapy an ideal treatment option to fight hot tumors. Hot tumors release pro-inflammatory cytokines which attract new immune cells. If PD-L1 inhibitors are used during immunotherapy, T-cells can naturally regain function and start fighting the tumor more rapidly and effectively. Patients with hot tumors generally have a better prognosis as their immune system is actively fighting the cancer. (Wang et al. 2023)
As opposed to hot tumors, cold tumors are immune-excluded or immune-deserts. There is lack of immune cell infiltration and low expression of immune checkpoint molecules which make its underlying mechanisms more complex and more difficult to treat.
Cold tumors often have an immunosuppressive microenvironment that prevents activation and recruitment of immune cells. High levels of immunosuppressive cells such as Tregs, MDSCs are observed while low levels of NK cells and activated lymphocytes are observed. For example, using PD-L1 inhibitors prove ineffective or barely effective since there are a plethora of immunosuppressants present which have eradicated immune cells from the tumor site almost entirely if not entirely. In some cases, cold tumors may also produce factors that inhibit immune cell function entirely. As a result of the above, cold tumors tend to have a worse prognosis as the immune system is deactivated. Cold tumors are less responsive to immunotherapy and thus, alternative treatment options are recommended. (Wang et al. 2023)
IV. IMMUNOTHERAPIES
Immunotherapy is a form of cancer treatment which works by harnessing the body’s own immune system to fight the disease, which is in this case, cancer. As discussed extensively in previous sections, cancer works to mask itself from the immune system and use it for its own advantage (for example, using macrophages to induce angiogenesis) (Joyce & Pollard, 2009). Immunotherapy involves reversing these changes brought about by the cancer and focusing on restoring our immune system to a stable state so that the tumor can no longer enter the escape state. Our goal here is to use the immune system to fight off the cancer and stop it from metastasizing further.
Immunotherapy often has a better outcome in tumors which already have presence of immune cells in the TME. Tumor-infiltrating lymphocytes or TILs are a sign that the immune system is responding to the tumor. People whose tumors contain TILs often do better than people whose tumors don’t contain them (National Cancer Institute, Immunotherapy for cancer 2024). This is due to the simple reason that available lymphocytes can be easily harnessed, which is the case in hot tumors. Cold tumors are immune deserts, which is what makes them notoriously hard to treat. There are almost no immune cells present in the TME which can be harnessed. To combat this issue, there is a race to make cold tumors hot.
A few of the most popular immunotherapy strategies include monoclonal antibody therapies, checkpoint inhibitors, CAR-T cells and vaccines. This section discusses various immunotherapy strategies, along with their effectiveness and mechanisms of action in treating different cancers.
A. Monoclonal Antibody Therapies (MABS)
Monoclonal antibody therapy involves taking lab made proteins, antibodies and molecules and introducing them into the TME via intravenous injection (IV) of the patient. Monoclonal antibodies act like natural antibodies in the sense that they can stimulate the immune system to fight harmful pathogens, including cancer cells (NCI, 2024). These antibodies are uniform (identical) and bind to a specific target antigen, which may only be expressed on pathogenetic or abnormal cells. This specificity allows monoclonal antibodies to target diseased cells while minimizing damage to healthy tissues. Essentially, monoclonal antibodies reverse the immunoediting carried out by the cancer. The goal is to reactivate the immune system and make it flag the cancerous tumor as ‘non-self’. They are a type of targeted cancer therapy, which means they are designed to interact with specific targets. Novel antibodies have also been found to target signaling pathways directly. Newer mAbs focus directly on proliferative pathways, acting like a downregulator for pathways such as EGFR, SRC among others. Other monoclonal antibodies bring T cells close to cancer cells, helping the immune cells kill the cancer cells. An example is blinatumomab, which binds to both CD19, a protein found on the surface of leukemia cells, and CD3, a protein on the surface of T cells (Zahavi & Weiner, 2020). This process helps the T cells get close enough to the cancer cells to respond to and kill them. The figure below (figure 5.) depicts how monoclonal antibodies work in the TME (Zahavi & Weiner, 2020).
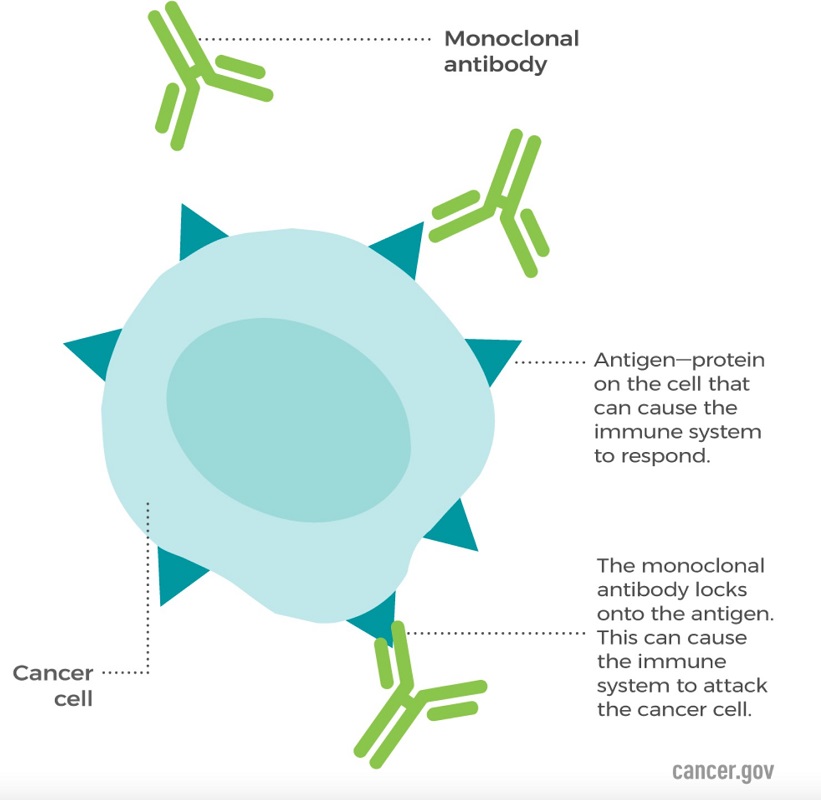
Figure 5. The Interaction Between Monoclonal Antibodies and Cancer Cells. Adapted from the National Cancer Institute, Monoclonal Antibodies (National Cancer Institute, 2024)
1) Mechanism of Action
Monoclonal Antibodies target antigens present on tumor cells due to their abnormal behavior. Tumor cells tend to synthesize abnormal proteins, interleukins and growth factors, called antigens. This, coupled with the MHC complex presentation, makes the cancerous cells depict certain antigens which can be targeted by artificial lab-made antibodies.
The main mechanism by which many antibodies induce tumor cell death is by the blockade of growth factor receptor signaling. Pro-tumor growth and survival signaling is inhibited when monoclonal antibodies bind their target growth factor receptors and manipulate their activation state or block ligand binding. For example, epidermal growth factor receptor (EGFR) is overexpressed by many different cancers and signaling via EGFR leads to tumor cell proliferation, migration, and invasion. Cetuximab, which is an anti-EGFR monoclonal antibody, induces apoptosis in tumor cells by blocking ligand binding and receptor dimerization (Zahavi & Weiner, 2020). Blocking the EGFR pathway will not only reduce proliferation but also make the tumor cells more sensitive to immune responses as they will not be able to enter the ‘escape’ phase as effectively.
The initial mAbs were limited not only in their utility but also in their targets. Early options focused on targeting soluble molecules (such as cytokines) (Malik, 2023). This however, presented a problem as targeting soluble molecules was difficult, and extremely cost intensive. This was not ideal as well, since sequestered soluble molecules can always be produced again. Cytokines play a crucial role in regulating the immune response. As is discussed in the previous sections, cancer cells immune edit and change certain interleukin and TNF levels, thereby hindering the immune response. Early mAbs reinstated certain cytokines and interleukins through antigen-binding allowing cells to express the correct factors for an immune response.
Novel agents have expanded to include a broader range of targets. For example, direct tumor elimination in oncology applications of mAbs can be accomplished through the inhibition of specific receptors or proteins on a cell which will interfere with essential functions such as dimerization, kinase activation, and signal cascade propagation. The dysfunction of these mechanisms can lead to reduced growth and eventual apoptosis of malignant cells (Zahavi & Weiner, 2020).
Antibodies work to recruit different molecules which may aid at the site of infection. The recruitment of the immune system (through cytokines, TNFs) is carried out by the Fc (fragment crystallizable) section of the mAb (figure 6.). Fc receptors can modulate the cell killing by antibody-dependent cellular cytotoxicity (ADCC) or by complement dependent cytotoxicity (CDC). ADCC involves the binding of monoclonal antibodies to antigens on the surface of target cells. The Fc region of the bound antibody can then interact with Fc receptors on the surface of immune effector cells, such as natural killer (NK) cells, macrophages, and neutrophils, thereby resulting in apoptosis and a coordinated immune response (Gedela, 2019). In addition, cytotoxic drugs can be conjugated to the Fc region leading to ADCC. In contrast, CDC is initiated when the Fc region of the monoclonal antibody, after binding to the target cell antigen, activates the complement system. This is a cascade of protein activations that ultimately form the membrane attack complex (MAC) usually in response to pathogenic infection (Hafeez & Scott, 2018).
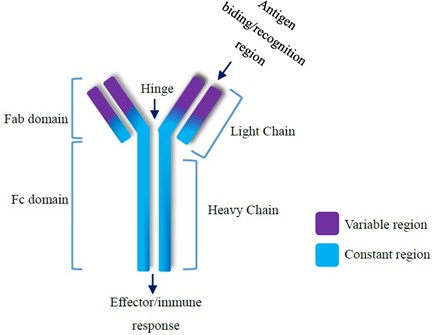
Figure 6. The Structure of a Monoclonal Antibody. Adapted from Saeed and Awan, Advances in Monoclonal Antibodies Production and Cancer Therapy, 2016 (Saeed and Awan, 2016).
2) Effectiveness and Current Applications
Monoclonal antibody therapy is an effective cancer treatment alone or in combination with other treatments. In most cases, monoclonal antibody treatment does not cure cancer. Many times cancer will return however, mAb is helpful in prolonging life without regression (Cleveland Clinic, 2024). When coupled with radiation therapy, mAb therapy proves to be extremely promising. Many mAb therapy drugs are FDA-approved and safe for usage to treat cancer.
For example, Rituximab is a successful mAb in the clinic, which targets the CD20 receptor on B lymphocytes, the antibody was found to be effective when coupled with standard-dose chemotherapy as a first line treatment. Patients given rituximab (with cyclophosphamide) showed efficacy results of 81% cancer removal. Chemotherapy alone showed an efficacy of 57%. It was observed that no toxicity changes were present as a result of coupling mAb and chemotherapy (Levene et al. 2005).
The advantage of mAb therapy is that the treatment is minimally invasive. The influence of external factors is limited. Our own immune system is harnessed, and that makes the side-effects and load on our body relatively limited.
3) Side Effects and other Shortcomings
Monoclonal antibody therapies are not a cure for cancer. The results of mAb therapies are only highly effective when given in combination with other existing therapies such as chemotherapy or radiation. Even then, the efficacy rates of cancer removal using mAb hover around 60%-80% tumor removal, making recurrence a possibility. Common side effects of mAbs include allergic reactions, such as hives (at the site of IV injection), flu-like symptoms, nausea, skin rashes and low blood pressure (Mayo Clinic, 2023). A major caveat with mAb therapy is the risk of cytokine storms and autoimmune disease, however this is rare (Tvedt et al. 2021). Lastly, and probably most importantly, mAb therapy is extremely expensive. The high price point of mAb makes it inaccessible, not widely available and limited in its dispersion and usage. According to one source, the price of receiving mAb therapy (including oncology and radiation) in the US was close to $96,731 per annum (Hernandez et al.
2018). High price points raise a host of ethical concerns including personalized therapy, need for equal access to all, and the need for better and more equitable forms of treatment.
B. Checkpoint Inhibitors
Immune checkpoints are receptors and ligands expressed on the cell surface of immune cells that are a normal part of the immune system. Immune checkpoints lead to the activation or repression of regulatory pathways associated with immune responses. They modulate the ‘level of response’ of the immune system and ensure that healthy cells are not affected (Pardoll, 2012). The two most prominent pathways of checkpoint inhibitors are the CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4) and PD-1/ PD-L1 (Programmed Death-Ligand 1) pathways both of which repress the activation of T cells (Shiravand et al. 2022).
Cancer immunoediting can manipulate the expression of PD-1/PD-L1 and CTAL-4 and dampen the immune response. Immune checkpoints engage when proteins on the surface of T cells bind to partner proteins on other cells, such as tumor cells. Tumor cells, by using immunoediting for its advantage, have the ability to send an ‘off’ signal to the T cells, preventing the immune system from destroying the cancer (NCI, 2022).
Immune checkpoint inhibitors are mAbs that interfere with the binding interaction between receptor and ligand associated with cancer immunoediting.This ensures that the T cells stay active and can fight the cancer, while evading the cancer’s mechanism to immuno-edit and escape (NCI, 2022). Since its introduction in 2011, seven new checkpoint inhibitors have been clinically approved for cancer treatment (Zhu & Wu, 2021). The following discusses checkpoint inhibitors further.
1) Mechanism of Action CTLA-4 Pathway
Cancer harnesses CTLA-4 to reduce the T cell response (NCI, 2022). This happens in a long chain of events starting from the MHC. A T cell first recognises an antigen presented by an antigen presenting cell (APC) via the MHC. A second signal is always required for full activation and response (figure 7) (Buchbinder & Desai, 2016). This second signal is achieved when CD28 (on the surface of T cells) binds to B7 molecules of APCs. CTLA-4, expressed on T cells, has a higher affinity of B7 molecules than CD28. When CTLA-4 binds to B7, it ‘outcompetes’ CD28, delivering an inhibitory signal to the T cell (Buchbinder & Desai, 2016). This reduces T cell proliferation and cytokine production, leading to a stunted immune response.
The interaction between APCs and T cells is well documented in literature, therefore, blocking CTLA-4 directly to prevent binding with B7 molecules is a strong target (Camacho, 2015). In the context of cancer, the tumor acts as the APC and drugs like ipilimumab block CTLA-4, this allows CD28 to continue providing the costimulatory signal, thereby sustaining T cell activation and enhancing the immune response against cancer cells.
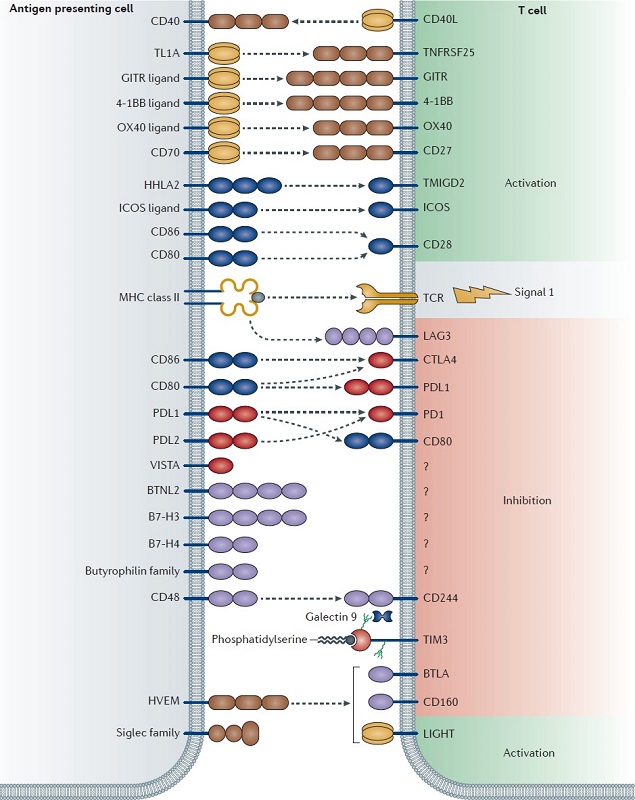
Figure 7. A Diagrammatic Representation of APC and T cell Interaction With Relation to Immune Checkpoint Inhibitors. Adapted from Mahoney et al, Combination cancer immunotherapy and new immunomodulatory targets (Mahoney et al. 2015)
2) PD-L1 Pathway
As discussed above, PD-L1 plays a crucial role in tumor survival and proliferation. PD-1 is an inhibitory receptor expressed on the surface of T cells (Han et al. 2020). Its primary ligands include PD-L1 and PD-L2, which can be expressed on cancer cells as well as other cells in the TME. When PD-1 binds to PD-L1 or PD-L2, an inhibitory signal is sent to the T cell, reducing its ability to kill target cells (Han et al. 2020). This pathway is a key mechanism by which tumors evade immune surveillance. Anti PD-1 drugs such as pembrolizumab and nivolumab directly block the interaction between PD-1 and its ligands, thereby inhibiting the pathway. These drugs restore the ability of T cells to recognise and destroy cancer cells (Zhang et al. 2021). The following figure (figure 8) depicts the mechanism of anti PD-L1 checkpoint inhibitors.
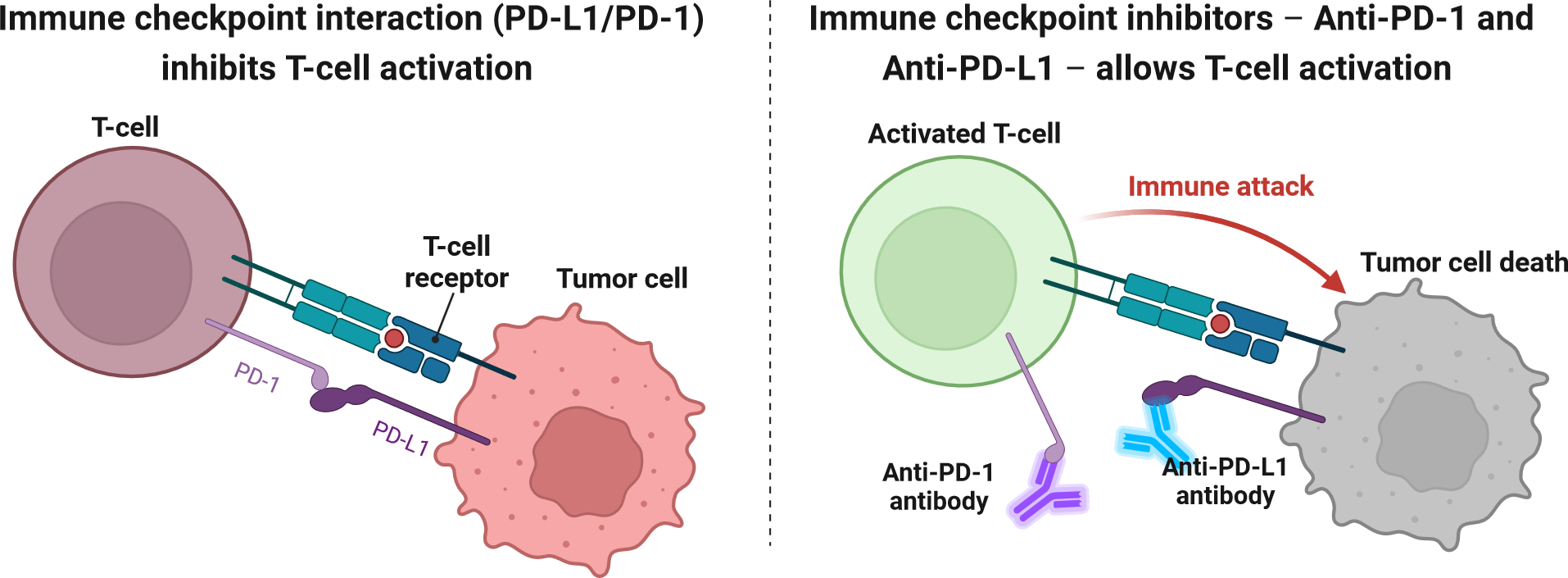
Figure 8. Mechanism of action of anti PD-L1 checkpoint inhibitors, adapted from Alexandra Ornelas, BioRender,Schematic diagram of the mechanism of action of Immune Checkpoint Inhibitors (ICI), (Ornelas, 2024)
In conclusion, checkpoint inhibitors are mAbs against proteins such as CTLA-4, PD-1, or PD-L1 By binding to these proteins, they prevent the inhibitory interactions between T cells and cancer cells or other cells in the tumor microenvironment, thereby preventing immune-escape.
3) Effectiveness and Current Applications
Similarly to mAbs, checkpoint inhibitors are not effective by themselves. Usually, they are given alongside other cancer remedies such as radiation and chemotherapy. Checkpoint inhibitors are administered through IV at the tumor site (Wikipedia, Ipilimumab 2024).
Ipilimumab, a CTLA-4 inhibitor, was the first ICI (Immune checkpoint inhibitor) to enter clinical use, approved in 2011 by the FDA following demonstration of an overall survival benefit in a randomized phase III trial in metastatic melanoma, a previously treatment-resistant, highly fatal malignancy with median survival less than 1 year (Tan et al. 2022). In modern immunotherapy at least 50% of patients, even with widespread metastatic disease including brain secondaries, appear to be cured (Tan et al. 2022). This is the only CTLA-4 inhibitor commonly used in standard practice, either as monotherapy or in combination with PD-1/PD-L1 inhibitors.
Durvalumab, A drug that binds to the protein PD-L1 to help immune cells kill cancer cells better and is used to treat different types of cancer. Durvalumab is used alone or with other drugs to treat adults with certain types of biliary tract cancer (including bile duct cancer and gallbladder cancer), endometrial cancer, hepatocellular carcinoma (a type of liver cancer), non-small cell lung cancer, and small cell lung cancer. It is also being studied in the treatment of other types of cancer. Durvalumab may block PD-L1 and help the immune system kill cancer cells. It is a type of monoclonal antibody and a type of immune checkpoint inhibitor (NCI, 2024). This PD-L1 checkpoint inhibitor has shown promising results as well. The following graph (figure 8.) shows its efficacy.
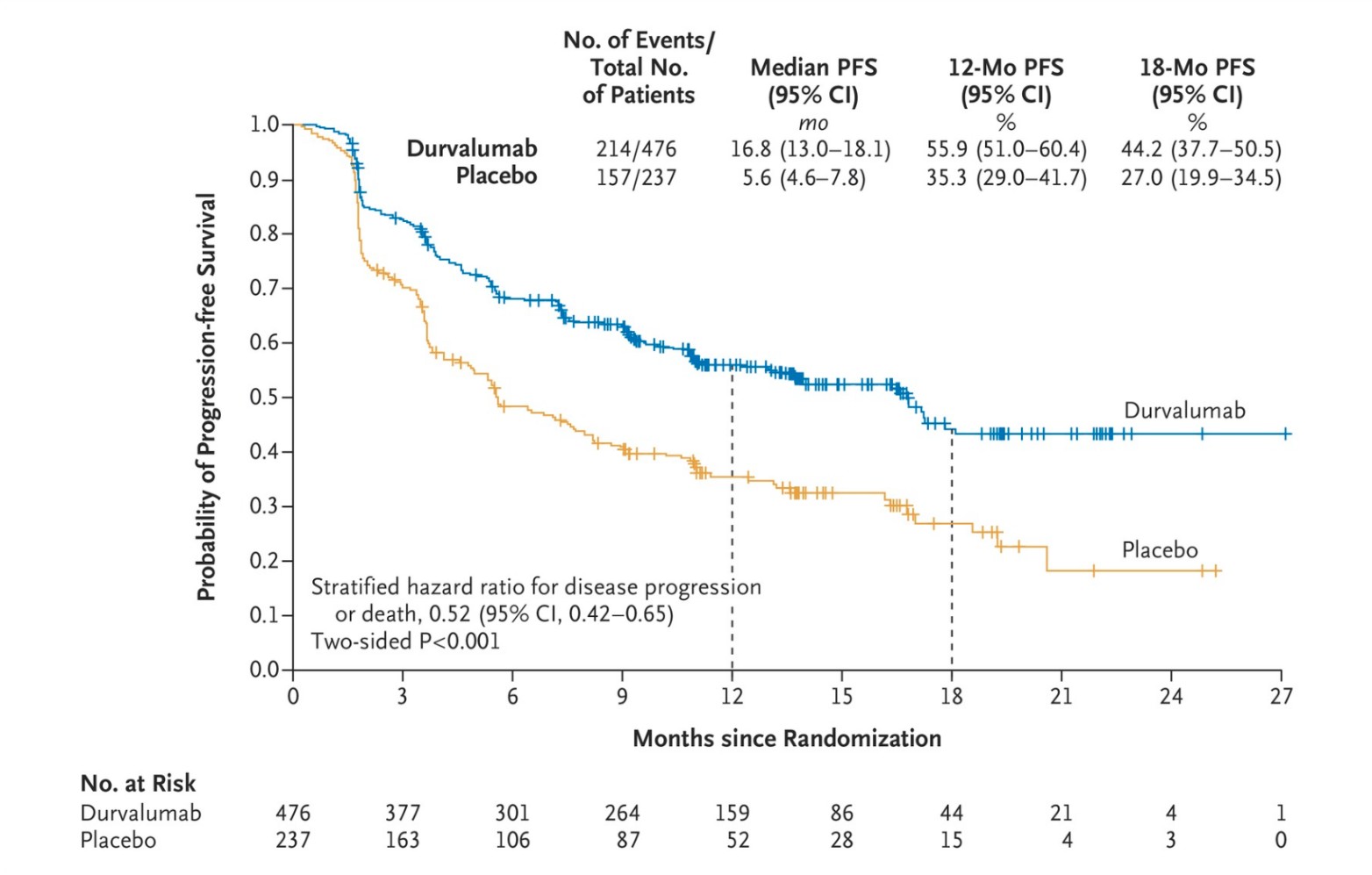
Figure 8. Efficacy of Durvalumab- probability of progression-free survival of placebo (5.6 months) versus durvalumab (16.8 months) after 2 rounds of chemotherapy/radiotherapy. Adapted from Antonia et al. The New England Journal of Medicine, 2017, Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer (Antonia et al. 2017)
4) Side Effects and Other Shortcomings
Similar to mAbs, ICIs do not show 100% efficacy in removing tumors. As is shown by the graph, despite Durvalumab treatment, the probability of progression-free survival hovers close to 40%-60%.
PD-1 and CTLA-4 prevent autoimmunity and limit immune activation to prevent bystander damage under physiological conditions. Therefore, Inhibition of these receptors is associated with a wide range of side effects that resemble autoimmune reactions (Seidel et al. 2018). 27.3% of patients treated with anti-CTLA-4 medication experienced notable side effects while 16.3% of patients treated with anti PD-1 medication experienced notable side effects. Even more patients were affected when treated with a combination of both (55%) (Seidel et al. 2018). Common side effects include diarrhea, fatigue, pruritus, rash, nausea and a decreased appetite. In more severe cases, patients experienced colitis, increased alanine aminotransferase levels, inflammation pneumonitis, and interstitial nephritis (Seidel et al. 2018).
These treatments tend to be extremely expensive, and are usually not covered by medical insurance due to their experimental and novel nature.
The price for one round of treatment of common ICIs hover around $10,000-$15,000, making them largely inaccessible (Paul et. al 2024).
C. Chimeric Antigen Receptor (Car) T Cell Therapy
CAR T cell therapy is a type of treatment in which a patient's T cells are genetically manipulated to attack cancer cells. In short, T cells are taken from a patient’s blood then the gene for a special receptor (Chimeric Antigen Receptor) that binds to a certain protein on the patient’s cancer cells is added to the T cells in the laboratory (NCI, 2024). Large numbers of the CAR T cells are grown in the laboratory and given to the patient by infusion. CAR T-cell therapy is used to treat certain blood cancers, and it is being studied in the treatment of other types of cancer (NCI, 2022).
Since 2017, six CAR T-cell therapies have been approved by the Food and Drug Administration (FDA). All are approved for the treatment of blood cancers, including lymphomas, some forms of leukemia, and, most recently, multiple myeloma (NCI, 2022).
As is discussed in earlier sections, cancer immune edits and downregulates certain proteins in order to stay undetected from the immune system. Certain antigens, which are normally expressed by ‘non-self’ cells, are not coded and expressed in cancer tumor cells (Mittal et al. 2014). Cancer blocks receptors on T-cells. CAR T cell therapy focuses on engineering cells to particularly target cancer antigens such as CD19 and BCMA (NCI, 2022). Due to cancer’s various immunoediting mechanisms, our T cells are unable to recognise these proteins. It is thus necessary to genetically engineer our T cells, adding specific receptors.
1) Mechanism of Action
T cells are collected from the patient or donor's blood through a procedure called leukapheresis (Zhang et al., 2017). The T cells are isolated and activated using methods like anti-CD3/anti-CD28 beads or growth factors like IL-2, which promotes their rapid growth. These steps ensure that the right T cell subsets are selected and prepared for modification (Zhang et al., 2017). The CAR gene is introduced into the T cells using viral vectors which integrate the CAR into the T cell's DNA (Hong et al. 2020). Other gene editing mechanisms include CRISPR-Cas9 (a revolutionary genome-editing tool that allows precise, targeted changes to DNA, enabling scientists to modify genes with high accuracy). The modified T cells are grown in bioreactors, such as the WAVE Bioreactor, G-Rex, or the fully integrated CliniMACS Prodigy system, which simplifies the process by performing multiple steps in one device (NCI 2022). After expanding to the necessary quantity, the CAR T cells are harvested, tested for quality, and then transfused back into the patient. Once this process has occurred, the CAR T cells can be employed to specifically target tumor cells through a complex mechanism.
Antigen recognition is the primary mechanism by which CAR T cells work. After the added chimeric antigen receptor recognises certain proteins (such as CD19 and BCMA) on tumor cells, anti-tumoral effects are elicited through the secretion of inflammatory cytokines (IL-2, IFN-γ and TNF-α) (Benmebarek et al., 2019). CAR T cells then induce apoptosis through TNF-related factors (Alnefaie et al., 2022). Tumor cell apoptosis can also be initiated via the activation and formation of the death signaling complex (DISC) leading to cell death mediated by mature caspase 3 (Nagata and Tanaka, 2017).
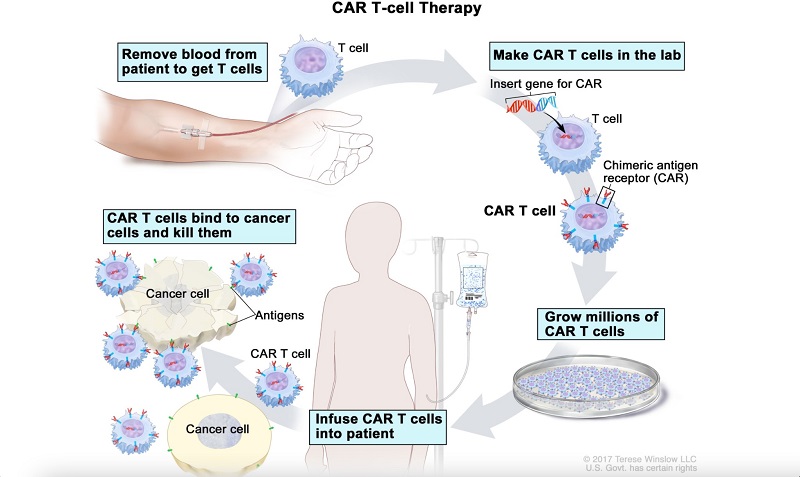
Figure 9. The Process of CAR T-cell Therapy Adapted from National Cancer Institute, 2024, CAR T-cell therapy (NCI, 2024)
Main structural components of the CAR include the single-chain variable fragment (scFv) of an antibody, which provides target specificity. There is a hinge and transmembrane region, along with a costimulatory domain and a T-cell activation domain (Brudno et al., 2018). Normal T-cells lack costimulatory domains. Co-stimulatory domains are derived from co-stimulatory receptors expressed on the surface of T cells. In the process of making CAR T cells, two costimulatory domains are introduced which are derived from different co-stimulatory proteins. These T cells are referred to ‘T cells redirected for universal cytokine-mediated killing (TRUCKS). Once activated, these CARs rapidly produce the desired chemokine (Brudno et al., 2018).
2) Effectiveness and Current Application
CAR T-cell therapy holds immense promise for the future of cancer treatment due to several key advantages. This innovative therapy has demonstrated significant success, particularly in treating blood cancers such as leukemia and lymphoma (Karkinos, Radhakrishnan, 2024). Notably, studies have reported remission rates as high as 80% in some cases, surpassing the outcomes typically achieved by traditional therapies (Karkinos, Radhakrishnan, 2024). Unlike chemotherapy or radiation, which indiscriminately target all rapidly dividing cells, CAR T cells are specifically programmed to attack a particular antigen on cancer cells. This targeted approach minimizes damage to healthy tissues and reduces the severity of side effects (National Cancer Institute (NCI, 2022). Moreover, CAR T cells are engineered to multiply and persist within the patient's body, providing long-term protection against cancer recurrence. They function as a living defense system, continuously patrolling the body to identify and eliminate cancer cells (NCI, 2022).
Long-term data collected over the past decade reveals that CD19-targeted CAR T cells can induce prolonged remissions in patients with B cell malignancies, often with minimal long-term toxicities. For some patients, this therapy is potentially curative. On the other hand, while remissions induced by BCMA-targeted CAR T cells tend to be shorter-lived, they also generally exhibit limited long-term toxicities (Cappell & Kochenderfer, 2023).
CAR T-cell therapy is a potent treatment option for patients with hematological malignancies, with long-term data demonstrating both robust efficacy and low overall toxicity levels. The highly durable remissions observed following CD19-targeted CAR T-cell therapy in patients with B cell lymphoma underscore the potential of this treatment modality to induce curative remissions in patients with chemotherapy-refractory malignancies.
3) Side Effects and Other Shortcomings
According to Penn Medicine, National Cancer Institute and Mayo Clinic, common side effects of CAR T cell therapy include CRS . Other complications include fever and chills, dizziness, lightheadedness, headaches, fatigue, pain in the muscles or joints, nausea, rapid heartbeat, low blood pressure, and difficulty breathing. In extreme cases, the nervous system may be negatively affected. Some patients have been shown to show confusion, tremors and a loss of balance and even consciousness.CAR T-cell therapy, while promising, has several notable shortcomings:
CAR T-cell therapy has shown significant success in treating certain types of blood cancers, such as B-cell malignancies, but its effectiveness in treating solid tumors remains limited. Solid tumors present challenges like a hostile tumor microenvironment and the lack of a well-defined target antigen, which can impede the efficacy of CAR T cells (Guzman et al., 2023)
As is common with most immunotherapies, CAR T-cell therapy is extremely expensive, with costs often exceeding hundreds of thousands of dollars per patient (Sterner & Sterner, 2021). This high price makes the therapy inaccessible to many patients, particularly in low- and middle-income countries. Additionally, the therapy requires highly specialized facilities and expertise, further limiting its availability. Simultaneously, CAR T-cell therapy involves a complex and time-consuming manufacturing process, where a patient’s T cells are harvested, genetically modified, and expanded before being reinfused. A lot of T cells may be lost during gene editing or while in culture. This process of generating CAR T-cells can take several weeks, during which the patient's condition may deteriorate, potentially making them ineligible for the therapy by the time it is ready. Lastly, the ever editing cancer cells may evolve to escape detection by CAR T cells, either by downregulating the targeted antigen or by acquiring mutations that render them invisible to the engineered T cells (Majzner & Mckall, 2020). This immune escape can lead to disease relapse and limits the long-term efficacy of the therapy.
D. Cancer Vaccines
Another promising avenue of cancer immunotherapies include cancer vaccines. The history of cancer vaccines dates back to the late 19th century when Dr. William Coley, known as the "father of immunotherapy," observed that bacterial infections sometimes led to tumor regression in cancer patients.
Coley began injecting patients with heat-killed bacteria, known as "Coley’s toxins," to stimulate the immune system to attack cancer cells (McCarthy, 2006). Although results varied, this laid the groundwork for immunotherapy.
In the mid-20th century, the discovery that certain viruses could cause cancer, such as the human papillomavirus (HPV) linked to cervical cancer, spurred the development of preventive cancer vaccines. The hepatitis B vaccine, introduced in the 1980s, was the first to prevent a virus-related cancer (liver cancer) (Frazer, 2019).
Therapeutic cancer vaccines, designed to treat existing cancers, emerged in the late 20th century. Despite initial setbacks, the approval of Sipuleucel-T (Provenge) by the FDA in 2010 for metastatic prostate cancer marked a significant milestone (Madan & Gulley, 2012).
The 21st century has seen advances in personalized mRNA cancer vaccines, particularly those targeting neoantigens (unique mutations in a patient’s tumor) (Li et al., 2023). These vaccines aim to elicit a robust immune response specifically against cancer cells. The recent success of mRNA vaccines for COVID-19 has also accelerated interest in using this technology for cancer vaccines, offering a promising new approach (Li et al., 2023). Today, research is focused on combining cancer vaccines with other immunotherapies, such as checkpoint inhibitors. Ongoing clinical trials continue to explore the potential of cancer vaccines in treating various cancers, signaling a promising future for this field (Li et al., 2023).
1) Mechanism of Action
Cancer vaccines are a type of immunotherapy that treats the cancer by strengthening the body’s natural defenses against cancer (NCI, 2019). Typically, cancer vaccines are not preventative. They are employed in patients who are already diagnosed with cancer. In a broad sense, cancer vaccines target tumor-associated antigens which are not present in normal cells. Treatment vaccines can help the immune system learn to recognise and react to these antigens and destroy the cancer cells that contain them (NCI, 2019). By mechanism, cancer vaccines are of two types, prophylactic and therapeutic.
2) Prophylactic Vaccines
Prophylactic cancer vaccines are designed to prevent cancer by priming the immune system to recognize and attack cancer-causing agents, typically viruses linked to cancer development (Crews et al., 2021). These vaccines primarily target oncogenic viruses, such as the human papillomavirus (HPV), associated with cervical and other cancers, and the hepatitis B virus (HBV), which is linked to liver cancer (Crews et al., 2021). The vaccines contain specific antigens, often derived from inactivated virus particles, recombinant proteins, or viral-like particles (VLPs). When administered, these antigens are recognized by antigen-presenting cells (APCs), like dendritic cells, which process the antigens and present them on their surface (Donaldson et al. 2018).
This antigen presentation triggers the activation of the immune response. Helper T cells (CD4+ T cells) recognize the antigens and activate cytotoxic T cells (CD8+ T cells) and B cells. The cytotoxic T cells then target and destroy cells infected by the virus, while B cells produce antibodies specific to the viral antigens (Crews et al., 2021). An essential aspect of this process is the formation of immune memory. Memory T cells and B cells specific to the viral antigens are generated and remain in the body long-term, providing a rapid and robust immune response if the body encounters the virus again (Wang et al. 2023).
By preventing the initial viral infections that could lead to cellular changes and tumor development, these vaccines significantly reduce the incidence of virus-induced cancers. For example, HPV vaccines have been shown to lower the risk of developing HPV-related cancers effectively (CDC 2021). Examples include Cervarix (GSK) (contains HPV-16/18), Gardasil (Merck) (contains HPV-6/11/16/18) and Hepatitis B vaccine.
3) Therapeutic Vaccines (Including mRNA vaccines)
Therapeutic cancer vaccines are an innovative form of immunotherapy designed to treat existing cancers by stimulating the patient’s immune system to recognize and attack cancer cells (Sobhani et al., 2022). Unlike prophylactic vaccines, which are intended to prevent disease, therapeutic vaccines aim to harness the body's immune defenses to target and destroy tumors that have already developed (Sobhani et al., 2022). These vaccines can be used on their own or in combination with other treatments like chemotherapy, radiation, or immune checkpoint inhibitors, enhancing the overall effectiveness of cancer therapy.
The mechanism of action of therapeutic cancer vaccines involves introducing specific cancer antigens into the body. These antigens are typically tumor-associated antigens (TAAs) present on the surface of cancer cells or neoantigens, which are unique to an individual’s tumor due to mutations (Fan et al., 2023). Once the vaccine is administered, antigen-presenting cells (APCs), such as dendritic cells, capture the antigens, process them, and present them on their surface in the context of major histocompatibility complex (MHC) molecules. This antigen presentation is crucial for activating the immune response.
Helper T cells (CD4+ T cells) recognize the antigens and, in turn, stimulate cytotoxic T cells (CD8+ T cells) to identify and destroy cancer cells expressing these antigens (Saxena et al., 2021). Additionally, B cells are activated to produce antibodies against the cancer-specific antigens, further bolstering the immune response.
The introduction of mRNA technology has significantly advanced the development of therapeutic cancer vaccines. mRNA vaccines operate by delivering the genetic instructions for specific cancer antigens directly into the patient’s cells. The process begins with the identification of suitable antigens that are overexpressed in cancer cells. Scientists then synthesize mRNA sequences that encode these antigens. To enhance the stability and efficiency of the mRNA, modifications are often made, ensuring it remains active in the body long enough to produce the target proteins.
Once synthesized, the mRNA is encapsulated in lipid nanoparticles (LNPs), which protect it from degradation and facilitate its delivery into cells (Sayour et al., 2024). After injection, these LNPs transport the mRNA into the patient’s cells, where it is translated into the target antigen protein. These proteins are then processed and displayed on the cell surface by MHC molecules, triggering an immune response. Cytotoxic T cells are activated to kill cells displaying the cancer antigen, while helper T cells and B cells coordinate a broader immune response, including antibody production (Hou et al., 2021). As this technology continues to evolve, it holds significant promise for improving outcomes in cancer treatment. Only sipuleucel-T (Provenge) is approved by the FDA (in 2010) for treatment of Prostate Cancer (Handy & Antonarakis, 2017). It is important to note that therapeutic vaccines work exogenously. Isolation of the APC using leukapheresis occurs first, after which mRNA may be introduced to complete the therapeutic cancer vaccine manufacturing (figure 10).
Sequencing individual patients' tumors to identify neoantigens enables the creation of highly targeted cancer vaccines (Rojas et al., 2023). These vaccines work by isolating the DNA or RNA from the tumor cells and sequencing it to identify mutations that create novel peptides, or neoantigens, which are not present in normal cells. Once identified, these neoantigens can be synthesized and used to create a personalized vaccine that trains the patient's immune system to recognize and attack cancer cells harboring these specific mutations. This approach holds significant promise because it tailors the vaccine to the genetic profile of the patient's tumor, potentially increasing its effectiveness while minimizing off-target effects on healthy cells (Rojas et al., 2023).
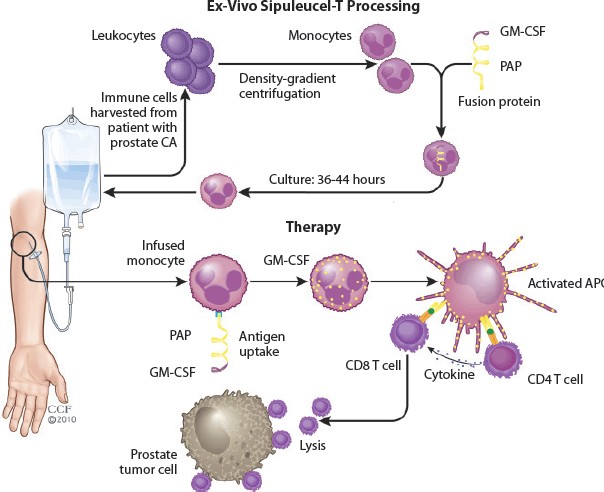
Figure 10. The Process of Therapeutic Cancer Vaccine Development and its Mechanism of Application. Adapted from Xu et al., 2019, Advances in Engineering Cells for Cancer Immunotherapy (Xu et al., 2019)
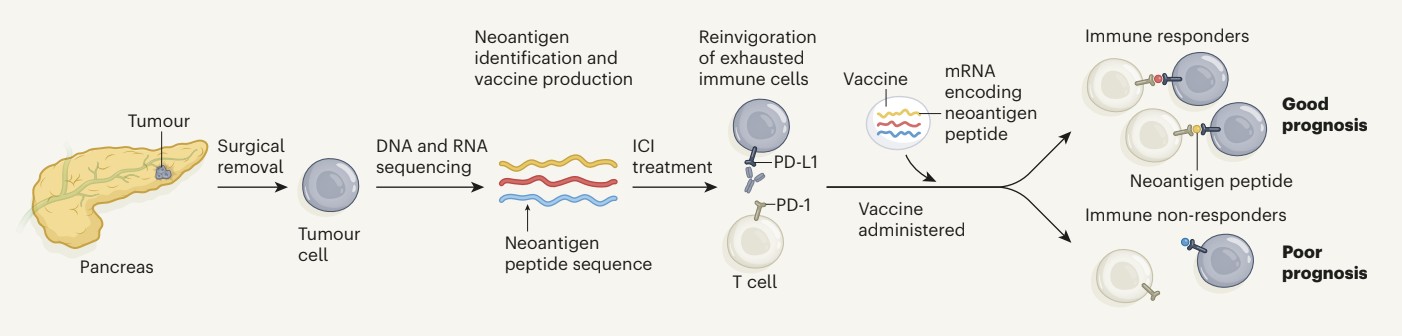
Figure 11. The Process of Making a Personalized Therapeutic Vaccine Following Tumor Sequencing from the Pancreas. Adapted from Huff and Zaidi, 2023, Vaccine boosts T cells that target pancreatic tumors, (Huff & Zaidi, 2023)
4) Effectiveness and Current Application
Cancer vaccines, encompassing both prophylactic and therapeutic types, represent a significant advancement in oncology, offering new avenues for preventing and treating various cancers. Their effectiveness and current applications are determined by the type of vaccine and the specific cancer they target, and they have shown considerable promise in both prevention and treatment settings.
5) Prophylactic Cancer Vaccines
The most prominent examples of this are the vaccines against human papillomavirus (HPV) and hepatitis B virus (HBV). The HPV vaccine, for instance, has demonstrated effectiveness in reducing the incidence of cervical cancer, as well as other HPV-related cancers, such as those of the throat and genitals (Brisson et al., 2020). Clinical data support the impact of these vaccines; for example, a study published in 2020 showed that HPV vaccination reduced cervical cancer rates by 87% in women aged 12-13 years at the time of vaccination, demonstrating a profound effect on public health (Brisson et al., 2020). Similarly, the HBV vaccine has been instrumental in decreasing the incidence of liver cancer, especially in regions where HBV infection is prevalent. Global HBV vaccination programs have led to an 84% reduction in HBV-related liver cancer rates among vaccinated cohorts, as reported by the World Health Organization (WHO) (Flores et al., 2022).
6) Therapeutic Cancer Vaccines
Therapeutic cancer vaccines have not yet achieved the same level of success as prophylactic vaccines, ongoing research and clinical trials are showing promising results (Elsheik et al. 2023). For example, in advanced melanoma, sipuleucel-T (Provenge) has been shown to improve survival rates by 10-20% when combined with checkpoint inhibitors (Redman et al., 2019). Another example with Provenge shows that in trials for prostate cancer, the median survival of patients was extended by approximately four months compared to the control group (Handy & Antonarakis, 2017).
Statistics from the American Association for Cancer Research (AACR) indicate that while therapeutic vaccines have shown a response rate of about 8-15% in some solid tumors, the success rate is significantly higher when these vaccines are used in combination with other therapies, such as checkpoint inhibitors, where response rates can exceed 40%. A lot of mRNA vaccines are currently in the clinical trial stage, and initial data proves to be increasingly promising.
7) Side Effects and Other Shortcomings
Prophylactic cancer vaccines, such as those for HPV and HBV, are generally well-tolerated, but they can cause mild side effects like pain at the injection site, fever, fatigue, and headaches. More serious side effects are rare but can include allergic reactions. Therapeutic cancer vaccines, which are designed to treat existing cancers by stimulating a stronger immune response, can cause more significant side effects. These may include flu-like symptoms, such as fever, chills, and fatigue, as well as more severe reactions like inflammation, autoimmune responses, and even cytokine release syndrome (CRS), where the immune system becomes overly activated, leading to potentially dangerous levels of inflammation throughout the body (CDC, Vaccine Safety, 2020).
The effectiveness of cancer vaccines, particularly therapeutic ones, can be another limitation. While prophylactic vaccines have proven highly effective in preventing virus-related cancers, therapeutic vaccines have had more mixed results (Kaczmarek et al., 2023).
For example, therapeutic vaccines may not work as well in patients with advanced or heavily immunosuppressed cancers because the tumor environment can suppress the immune response, reducing the vaccine's effectiveness (Kaczmarek et al., 2023). In some cases, the immune system might not recognize the cancer antigens well enough, leading to a weak or insufficient response to the vaccine.
Another significant shortcoming of cancer vaccines, particularly therapeutic ones, is their cost and accessibility. Developing and manufacturing these vaccines, especially personalized therapeutic vaccines like those based on neoantigens, is complex and expensive. The process involves sequencing the patient’s tumor to identify unique mutations, designing a vaccine tailored to those mutations, and producing it under stringent conditions. This personalized approach can lead to costs that run into tens or even hundreds of thousands of dollars per treatment course, making them prohibitively expensive for many patients. For example, the cost of some therapeutic cancer vaccines can exceed $100,000, limiting their accessibility to patients without adequate insurance or financial resources (Jönsson & Wilking, 2012)
The development of cancer vaccines is still a relatively new field, meaning that long-term data on their effectiveness and potential risks are limited. Additionally, the immune system’s complexity means that not all patients will respond to these vaccines in the same way, leading to variability in outcomes.
V. DISCUSSION
Immunotherapy has revolutionized cancer treatment by harnessing the body’s immune system to fight cancer cells. The development of various immunotherapies, including monoclonal antibodies (mAbs), immune checkpoint inhibitors (ICIs), CAR T-cell therapy, and cancer vaccines, has expanded treatment options for patients with different types of cancer. Each of these approaches has shown significant promise, but they also come with unique advantages, disadvantages, and challenges that must be addressed to optimize their use.
Monoclonal antibodies (mAbs) have been at the forefront of immunotherapy due to their ability to target specific antigens on cancer cells. They can directly kill cancer cells, block growth signals, or deliver cytotoxic agents to tumor sites. One of the key advantages of mAbs is their specificity, which minimizes damage to healthy tissues. However, resistance can develop over time, and not all patients respond to mAb therapy. Additionally, mAbs can cause significant side effects, including infusion reactions and cardiotoxicity. Despite these drawbacks, mAbs have proven to be a cornerstone of cancer therapy, particularly in treating cancers like breast cancer and lymphoma.
Immune checkpoint inhibitors (ICIs) have transformed the treatment landscape for cancers like melanoma, lung cancer, and renal cell carcinoma by blocking proteins that inhibit immune responses against tumors. These inhibitors, such as PD-1/PD-L1 and CTLA-4 blockers, have demonstrated the potential to produce durable responses and, in some cases, long-term remissions. However, ICIs are not effective for all patients, and they can trigger immune-related adverse events, including colitis, pneumonitis, and endocrinopathies. These side effects result from the overactivation of the immune system and can be severe, necessitating careful patient selection and monitoring during treatment.
CAR T-cell therapy represents a more personalized approach, where a patient’s T cells are engineered to express chimeric antigen receptors (CARs) that target specific cancer antigens. This therapy has shown remarkable success in treating hematological malignancies like certain types of leukemia and lymphoma, with some patients achieving complete remission. However, CAR T-cell therapy also presents significant challenges, including severe side effects like cytokine release syndrome (CRS) and neurotoxicity. Additionally, its effectiveness in solid tumors has been limited, and the manufacturing process is complex and costly, making it one of the most expensive cancer treatments available.
Cancer vaccines, including both prophylactic and therapeutic types, offer another avenue for immunotherapy. Prophylactic vaccines, like those against HPV and HBV, have been highly successful in preventing virus-related cancers. Therapeutic cancer vaccines aim to stimulate the immune system to attack existing tumors, though their effectiveness has been more variable. While vaccines are generally well-tolerated, their development and application can be limited by the need to identify specific tumor antigens and the challenge of overcoming the immunosuppressive tumor microenvironment.
A common issue across all these immunotherapies is their high cost. Treatments like CAR T-cell therapy and immune checkpoint inhibitors are prohibitively expensive for many patients, with costs often exceeding $100,000 per treatment course. This raises significant concerns about accessibility and equity in cancer care. In my opinion, while the clinical potential of these therapies is undeniable, there is an urgent need to develop strategies that can lower the costs and make these life-saving treatments more accessible to a broader population. This might include improving manufacturing processes, developing biosimilars, or implementing policy measures to control pricing.
In addition to the established immunotherapies, oncolytic viruses represent a novel and emerging approach. These viruses are engineered to selectively infect and kill cancer cells while stimulating an immune response against the tumor. While still in the early stages of clinical development, oncolytic viruses offer the potential to complement existing immunotherapies and overcome some of their limitations.
In evaluating the different immunotherapies, it is challenging to declare a single best approach, as their effectiveness depends on the type of cancer and the individual patient’s condition. However, immune checkpoint inhibitors have arguably made the most significant impact in recent years, particularly for patients with melanoma and lung cancer, offering long-term survival benefits that were previously unattainable. The future of cancer treatment likely lies in combining these therapies, leveraging the strengths of each while minimizing their respective weaknesses to provide the most effective and personalized care for cancer patients.
VI. ACKNOWLEDGEMENT
Thank you for the guidance of mentor Matthew Hill from The University of Cambridge in the development of this research paper.
References
[1] (US), N.I. of H. (1970) Understanding cancer, NIH Curriculum Supplement Series [Internet]. Available at: https://www.ncbi.nlm.nih.gov/books /NBK20362/#:~:text=Cancer%2C%20then%2C%20is%20a%20disease,an%20in dividual%20experiences%20as%20cancer [2] Alnefaie, A. et al. (2022) Chimeric antigen receptor T-cells: An overview of concepts, applications, limitations, and proposed solutions, Frontiers in bioengineering and biotechnology. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC92569 91/#:~:text=The%20mechanism%20 of%20action%20of,functi on%20via%20perforin%20and%20granzyme%20( [3] Anderson, N. and Simon, M. (2021) The tumor microenvironment, CB. Available at: https://pubmed.ncbi.nlm.nih.gov/32810447/ [4] Antonia, S. (2017) Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer | Nejm, New England Journal of Medicine . Available at: https://www.nejm.org/doi/full/10.1056/NEJMoa1709937 [5] Awan, S. and Saeed, A. (2016) Advances in monoclonal antibodies production and cancer therapy, MOJ Immunology. Available at: [6] https://medcraveonline.com/MOJI/advances-in-monoclonal-antibodies-production-and-cancer-therapy.html [7] Binnewies, M. et al. (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy, Nature News. Available at: https://www.nature.com/articles/s41591-018-0014-x [8] Brabletz, T. et al. (2018) EMT in cancer, Nature News. Available at: https://www.nature.com/articles/nrc.2017.118 [9] Bray, F. et al. (2022) Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries - bray - CA: A cancer journal for Clinicians - Wiley Online Library. Available at: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21834 [10] Brown, J.S. et al. (2023) Updating the definition of cancer, Molecular cancer research?: MCR. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10618731/ [11] Buchbinder, E.I. and Desai, A. (2016) CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition, American journal of clinical oncology. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4892769/ [12] Camacho, L.H. (2015) CTLA-4 blockade with ipilimumab: Biology, safety, efficacy, and future considerations, Cancer medicine. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4430259/ [13] Cancer survival statistics (2023) Cancer Research UK. Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/survival#heading-One [14] Canfell, K., Kim, J. and Brisson, M. (2020) Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low-income and lower-middle-income countries - the Lancet, Lancet. Available at: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30068-4/fulltext [15] Cappell, K.M. and Kochenderfer, J.N. (2023) Long-term outcomes following car T cell therapy: What we know so far, Nature News. Available at: https://www.nature.com/articles/s41571-023-00754-1 [16] Centre for Disease Control (CDC) (2020) Safety information for HPV vaccine, Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html [17] Centre for Disease Control (CDC) (2021) HPV vaccination: What everyone should know, Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/vaccines/vpd/hpv/public/index.html#:~:text=HPV%20vaccination%20is%20recommended%20a t,cause%20cancer%20later%20in%20life. [18] Cornel, A.M., Mimpen, I.L. and Nierkens, S. (2020) MHC class I downregulation in cancer: Underlying mechanisms and potential targets for cancer immunotherapy, Cancers. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7409324/ [19] Crews, D.W., Dombroski, J.A. and King, M.R. (2021) Prophylactic cancer vaccines engineered to elicit specific adaptive immune response, Frontiers in oncology. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8044825/ [20] Dilley, R.L. and Greenberg, R.A. (2015) Alternative telomere maintenance and cancer, Trends in cancer. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4669901/ [21] Donaldson, B. et al. (2018) Virus-like particle vaccines: Immunology and formulation for clinical translation, Expert review of vaccines. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7103734/ [22] Elsheikh, R., Makram, A.M. and Huy, N.T. (2023) Therapeutic cancer vaccines and their future implications, Vaccines. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10052542/ [23] Fan, T. et al. (2023) Therapeutic cancer vaccines: Advancements, challenges, and prospects, Nature News. Available at: https://www.nature.com/articles/s41392-023-01674-3 [24] Flores, J.E. et al. (2022) The global impact of hepatitis B vaccination on hepatocellular carcinoma, Vaccines. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9144632/ [25] Frazer, I.H. (2019) The HPV vaccine story, ACS pharmacology & translational science. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7089001/ [26] Gonzalez, H., Hagerling, C. and Werb, Z. (2018) Roles of the immune system in cancer: From tumor initiation to metastatic progression, Genes & development. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6169832/ [27] Goodman, S.R. (2020) Cell adhesion and the extracellular matrix, Goodman’s Medical Cell Biology (Fourth Edition). Available at: https://www.sciencedirect.com/science/article/abs/pii/B9780128179277000077 [28] Guzman, G. et al. (2023) Car-T therapies in solid tumors: Opportunities and challenges, Current oncology reports. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10110629/ [29] Han, Y., Liu, D. and Li, L. (2020) PD-1/PD-L1 pathway: Current researches in cancer, American journal of cancer research. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7136921/ [30] Handy, C.E. and Antonarakis, E.S. (2018a) Sipuleucel-T for the treatment of prostate cancer: Novel insights and future directions, Future oncology (London, England). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5925432/ [31] Handy, C.E. and Antonarakis, E.S. (2018b) Sipuleucel-T for the treatment of prostate cancer: Novel insights and future directions, Future oncology (London, England). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5925432/#:~:text=Sipuleucel%2DT%20is%20the%20only%20appro ved%20cellular%20immunotherapy%20in%20prostate,disease%20and%20no%20visceral%20metastases. [32] Hannahan, D. and Weinberg, R.A. (2011) Hallmarks of cancer: The next generation, Cell. Available at: https://pubmed.ncbi.nlm.nih.gov/21376230/ [33] Hernandez, I. et al. (2018) Pricing of monoclonal antibody therapies: Higher if used for cancer?, The American journal of managed care. Available at: https://pubmed.ncbi.nlm.nih.gov/29461857/ [34] Hong, M., Club, J. and Chen, Y. (2020) Engineering car-T cells for next-generation cancer therapy, Cancer Cell. Available at: https://www.sciencedirect.com/science/article/pii/S1535610820303664 [35] Hou, X. et al. (2021) Lipid nanoparticles for mrna delivery, Nature News. Available at: https://www.nature.com/articles/s41578-021-00358-0 [36] Hu, J. et al. (2021) Targeting mutant p53 for cancer therapy: Direct and Indirect Strategies, Journal of hematology & oncology. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8480024/ [37] Huff, A.L. and Zaidi, N. (2023) Vaccine boosts T cells that target pancreatic tumours, Nature News. Available at: https://www.nature.com/articles/d41586-023-01526-8#:~:text=Efforts%20to%20tackle%20pancreatic%20cancer,imm une%20responses%20to%20such%20tumours. (Accessed: 15 August 2024). [38] Ipilimumab (2024) Wikipedia. Available at: https://en.wikipedia.org/wiki/Ipilimumab#:~:text=Ipilimumab%2 0binds%20to%20CTLA% 2D4,number%20attacking %20a%20single%20antigen. [39] Janeway, C.A. and Jr (1970) The major histocompatibility complex and its functions, Immunobiology: The Immune System in Health and Disease. 5th edition. Available at: https://www.ncbi.nlm.nih.gov/books/NBK27156/ [40] Joyce, J. and Pollard, J. (2008) Microenvironmental regulation of metastasis, Nature reviews. Cancer. Available at: https://pubmed.ncbi.nlm.nih.gov/19279573/ [41] Jönsson, B. and Wilking, N. (2012) Cancer vaccines and Immunotherapeutics: Challenges for pricing, reimbursement and Market Access, Human vaccines & immunotherapeutics. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3579921/#:~:text=With%20an%20estimated%20cost%20of,life%20y ear%20gained%20is%20%24280%2C000. [42] Kaczmarek, M. et al. (2023) Cancer vaccine therapeutics: Limitations and effectiveness-A literature review, Cells. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10486481/ [43] Levene, A.P., Singh, G. and Palmieri, C. (2005) Therapeutic monoclonal antibodies in oncology, Journal of the Royal Society of Medicine. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1079437/#:~:text=The%20antibody%20has%20also%20been,compar ed%20with%2057%25%20in%20the [44] Li, X. et al. (2023) Neoantigen Cancer Vaccines: A new star on the Horizon, Cancer biology & medicine. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11033713/ [45] Li, Y. et al. (2023) MRNA vaccine in cancer therapy: Current advance and future outlook, Clinical and translational medicine. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10447885/ [46] Liu, Z.-L. et al. (2023) Angiogenic signaling pathways and anti-angiogenic therapy for cancer, Nature News. Available at: https://www.nature.com/articles/s41392-023-01460-1 [47] Madan, R.A. and Gulley, J.L. (2011) Sipuleucel-T: Harbinger of a new age of therapeutics for prostate cancer, Expert review of vaccines. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3460263/ [48] Mahoney, K., Freeman, G. and Rennert, P. (2015) Combination cancer immunotherapy and new immunomodulatory targets, Nature reviews. Drug discovery. Available at: https://pubmed.ncbi.nlm.nih.gov/26228759/ [49] Majzner, R.G. and Mackall, C.L. (2018) Tumor antigen escape from car T-cell therapy, American Association for Cancer Research. Available at: [50] https://aacrjournals.org/cancerdiscovery/article/8/10/1219/6227/Tumor-Antigen-Escape-from-CAR-T-cell# [51] Malik, B. (2023) Understanding how monoclonal antibodies work, StatPearls [Internet]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK572118/ [52] Mattiuzzi, C. and Lippi, G. (2019) Current cancer epidemiology, Journal of epidemiology and global health. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7310786/ [53] McCarthy, E.F. (2006) The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas, The Iowa orthopaedic journal. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1888599/ [54] McKinstry and Swain, S.L. (2010) Antigen-presenting cell, Antigen-Presenting Cell - an overview | ScienceDirect Topics. Available at: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/antigen-presenting-cell#:~:text=The%20pr oteins%20that%20form%20the,of%20the%20innate%20immune%20system. 2024). [55] MHC & Antigen presentation (2018) Immunopaedia. Available at: https://www.immunopaedia.org.za/immunology/basics/4-mhc-antigen-presentation/ [56] Mittal, D. et al. (2014) New insights into cancer immunoediting and its three component phases--elimination, Equilibrium and Escape, Current opinion in immunology. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4388310/ [57] Monoclonal antibodies (no date a) NCI. Available at:https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/monoclonal-antibodies [58] National Cancer Institute (2019) Cancer treatment vaccines - immunotherapy, Immunotherapy - NCI. Available at: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/cancer-treatment-vaccines [59] National Cancer Institute (2021) What is cancer?, NCI. Available at: https://www.cancer.gov/about-cancer/understanding/what-is-cancer [60] National Cancer Institute (2022) Car T cells: Engineering immune cells to treat cancer, CAR T Cells: Engineering Immune Cells to Treat Cancer - NCI. Available at: https://www.cancer.gov/about-cancer/treatment/research/car-t-cells#:~:text=The%20CAR%20T%2Dcell%20therapies ,B%20cells%2C%20C D19%20or %20BCMA. [61] National Cancer Institute (2024) Car T cells: Engineering immune cells to treat cancer, CAR T Cells: Engineering Immune Cells to Treat Cancer - NCI. Available at: https://www.cancer.gov/about-cancer/treatment/research/car-t-cells [62] NCI (2022) Immune checkpoint inhibitors, NCI. Available at: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/checkpoint-inhibitors [63] Nevins, J.R. (2001) The RB/E2F pathway and cancer, OUP Academic. Available at: https://academic.oup.com/hmg/article-abstract/10/7/699/558545?redirectedFrom=PDF . [64] Oda, K. et al. (2005) A comprehensive pathway map of epidermal growth factor receptor signaling, Molecular systems biology. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1681468/#:~:text=The%20epidermal%20growth%20factor%20recept or,and%20differentiation%20in%20mammalian%20cells [65] O’Donnell, J., Smyth and Teng, M. (2019) Cancer immunoediting and resistance to T cell-based immunotherapy, Nature reviews. Clinical oncology. Available at: https://pubmed.ncbi.nlm.nih.gov/30523282/ [66] Pardi, N. et al. (2018) MRNA vaccines - a new era in Vaccinology, Nature reviews. Drug discovery. Available at: https://pubmed.ncbi.nlm.nih.gov/29326426/ [67] Pardoll, D.M. (2012a) The blockade of immune checkpoints in cancer immunotherapy, Nature reviews. Cancer. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4856023/ [68] Pardoll, D.M. (2012b) The blockade of immune checkpoints in cancer immunotherapy, Nature reviews. Cancer. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4856023/ [69] Paul, J., Mitchell, A. and Rome, B. (2023) Trends in prices of checkpoint inhibitors in the US, 2016-2023, Trends in prices of checkpoint inhibitors in the US, 2016-2023. Available at: https://ascopubs.org/doi/10.1200/JCO.2024.42.16_suppl.11075 [70] Penn Medicine (2024) Pennmedicine.org. Available at: [71] https://www.pennmedicine.org/cancer/navigating-cancer-care/treatment-types/immunotherapy/what-is-car-t-therapy [72] Radhakrishnan, S. (2024) Why is car T cell therapy seen as the future of cancer treatment?, Karkinos Healthcare. Available at: [73] https://www.karkinos.in/why-is-car-t-cell-therapy-seen-as-the-future-of-cancer-treatment/#:~:text=Solid%20tumour% 20application%3A%20CAR%20T,activity%20of%20these%20engineered%20cells. [74] Redman, J.M., Gulley, J.L. and Madan, R.A. (2017) Combining immunotherapies for the treatment of prostate cancer, Urologic oncology. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6599516/ [75] Roche, J. (2018) The epithelial-to-mesenchymal transition in cancer, Cancers. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5836084/ [76] Rojas, L.A. et al. (2023) Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer, Nature News. Available at: https://www.nature.com/articles/s41586-023-06063-y (Accessed: 15 August 2024). [77] Saxena, M. et al. (2021) Therapeutic cancer vaccines, Nature News. Available at: https://www.nature.com/articles/s41568-021-00346-0 [78] Sayour, E.J. et al. (2024) Cancer mrna vaccines: Clinical advances and future opportunities, Nature News. Available at: https://www.nature.com/articles/s41571-024-00902-1 [79] Schematic diagram of the mechanism of action of immune checkpoint inhibitors (ICI).: BioRender science templates (no date a) Schematic diagram of the mechanism of action of Immune Checkpoint Inhibitors (ICI). | BioRender Science Templates. Available at: [80] https://www.biorender.com/template/schematic-diagram-of-the-mechanism-of-action-of-immune-checkpoint-inhibitor s-ici [81] Schematic diagram of the mechanism of action of immune checkpoint inhibitors (ICI).: BioRender science templates (no date b) Schematic diagram of the mechanism of action of Immune Checkpoint Inhibitors (ICI). | BioRender Science Templates. Available at: [82] https://www.biorender.com/template/schematic-diagram-of-the-mechanism-of-action-of-immune-checkpoint-inhibitor s-ici [83] ScienceDirect (2019) Single-chain variable fragment, Single-Chain Variable Fragment - an overview | ScienceDirect Topics. Available at: https://www.sciencedirect.com/topics/neuroscience/single-chain-variable-fragment [84] Scott, A. and Hafeez, U. (2018) Complement dependent cytotoxicity, Complement Dependent Cytotoxicity - an overview | ScienceDirect Topics. Available at: https://www.sciencedirect.com/topics/neuroscience/complement-dependent-cytotoxicity [85] Shiravand, Y. et al. (2022) Immune checkpoint inhibitors in cancer therapy, Current oncology (Toronto, Ont.). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9139602/ [86] Sobhani, N. et al. (2022) Therapeutic cancer vaccines: From biological mechanisms and engineering to ongoing clinical trials, Cancer treatment reviews. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9217071/ [87] Sterner, R.C. and Sterner, R.M. (2021) Car-T cell therapy: Current limitations and potential strategies, Nature News. Available at: https://www.nature.com/articles/s41408-021-00459-7 [88] Tan, S. et al. (2022) Immune checkpoint inhibitor therapy in oncology: Current uses and future directions: JACC: Cardiooncology state-of-the-art review, JACC. CardioOncology. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9830229/ [89] Vesely, M.D. and Schreiber, R.D. (2013) Cancer immunoediting: Antigens, mechanisms, and implications to cancer immunotherapy, Annals of the New York Academy of Sciences. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3648872/ [90] Voudson, K. and Prives, C. (2009) Blinded by the light: The growing complexity of p53, Cell. Available at: https://pubmed.ncbi.nlm.nih.gov/19410540/ [91] Wang, Lianjie et al. (2023) Hot and cold tumors: Immunological features and the therapeutic strategies, MedComm. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10458686/ [92] Wang, Lulu et al. (2023) T cell immune memory after COVID-19 and Vaccination, BMJ medicine. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10668147/ [93] Wojtukiewicz, M.Z. et al. (2021) Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for Internists and general practitioners, Cancer metastasis reviews. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8556173/ [94] Wu, S. and Zhu, H. (2021) Immune checkpoint inhibitor, Immune Checkpoint Inhibitor - an overview | ScienceDirect Topics. Available at: https://www.sciencedirect.com/topics/medicine-and-dentistry/immune-checkpoint-inhibitor [95] Xu, X., Shen, S. and Mo, R. (2019) Advances in Engineering Cells for Cancer Immunotherapy, Research Gate. Available at: [96] https://www.researchgate.net/figure/Schematic-of-the-artificially-reprogrammed-HIONMac-for-cancer-therapy-Repri nted-with_fig5_336874953 [97] Zahavi, D. and Weiner, L. (2020) Monoclonal antibodies in cancer therapy, Antibodies (Basel, Switzerland). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7551545/ [98] Zhang, C. et al. (2017) Engineering car-T cells, Biomarker research. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5482931/ [99] Zhang, Y. et al. (2021) PD-1 immune checkpoint inhibitor therapy malignant tumor based on monotherapy and combined treatment research, Technology in cancer research & treatment. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8093614/
Copyright
Copyright © 2024 Arhaan Kohli. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64285
Publish Date : 2024-09-19
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

