Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Review of New Antibiotics for Multidrug Resistant Bacterial Strains
Authors: Aarti S. Khobragade, Ajay P. Patil, Shubham B. Yadav, Dr. Rupali Tasgaonkar, Vishal T. Khandebarad
DOI Link: https://doi.org/10.22214/ijraset.2023.48972
Certificate: View Certificate
Abstract
Antibiotics usually referred to as antimicrobial medications, are medicines that combat bacterial infections. The greatest threat to the public\'s health comes from germs from clinical and non-clinical environments that are increasingly resistant to current antibiotics. One of the main contributors to antimicrobial resistance is the number of antibiotics that are generally consumed in the population. There is a lot of unofficial information concerning the misuse of antibiotics, their availability over the counter, and their dose, but there is very little concrete proof of local customs. The development of new antibiotics with the lowest level of microbial resistance is always made possible by the aforementioned facts. [1] The goal of the current investigation is to examine the troubling issue of antibiotic resistance and the creation of bacterial strains that are resistant to many drugs, both of which are now prevalent in hospitals and pose a threat to the global effort to control infectious diseases. Possible tactics to stop antibiotic resistance are analyzed after a thorough analysis of these occurrences and the numerous mechanisms that lead some bacteria to become resistant to particular medications that were once successful in treating diseases brought on by the same pathogens. [2]
Introduction
I. INTRODUCTION
The earlier definition of an antibiotic was a chemical substance that is produced by microorganism and, in dilute solutions can inhibit growth of, and even destroys other microorganisms. This definition now has been expanded to include similar inhibitory substances that are produced by plants, marine organisms, and total- or semisynthetic procedures. Since the discovery of penicillin by Alexander Fleming in 1928, thousands of antibiotics have been isolated and identified; some have been found to be of value in treatment of infectious diseases. They differ markedly in physicochemical and pharmacological properties, antimicrobial spectra, Mechanism of action. [1] By subjecting them to penicillin concentrations that are insufficient to kill the microorganisms, it is simple to breed penicillin resistance in germs in a lab setting. However, there is the risk that the uninformed man may easily under-dose himself and, by exposing his microbes to no antibiotics, cause the inevitable phenomenon of antibiotic resistance, which has already been observed in laboratories, to occur. Fleming's predictions came true: improper use and occasionally outright abuse of antibiotics hastens the emergence and spread of germs that are resistant to them. [2]
Unfortunately, the dramatic worldwide rise of bacterial pathogens resistant to antibacterial agents cannot be counteracted by the current low development pace of therapeutics with new mode(s) of action. The bulk of the antibiotics that have been licensed over the past 40 years has actually been derived from already-known chemical structures, and the most recent novel class of antibiotics was only recently identified in the 1980s. [3]
The overuse of antibiotics is one of the factors contributing to the growing number of antimicrobial-resistant bacterial infections. According to the CDC (Centers for Disease Control and Prevention), outpatient antibiotic overuse in the USA is a particular problem in the Southeast. [1]
II. ANTIBIOTIC RESISTANCE
It is the ability of microorganisms to withstand the effect of an antibiotic. Antibiotic resistance naturally develops via natural selection through random mutation and plasma exchange between bacteria of the same species. Antibiotic resistance can also be introduced officially into a microorganism through transformation protocols. [1]
III. CAUSES OF ANTIBIOTIC RESISTANCE
Antibiotic resistance develops naturally in bacteria. However, the emergence and spread of resistance may be accelerated by human activities. This can happen: [4]
- When human and animal health professionals overprescribe antibiotics.
- When people don't take their antibiotics as directed.
- Due to a lack of infection prevention and control measures and inadequate hygiene e.g., not washing hands properly.
- Due to people traveling around the world, spreading resistant bacteria.
- Antibiotic use exacerbates antimicrobial resistance (AMR), a natural phenomenon in microorganisms. Antibiotic-resistant bacteria can develop when they: [4]
- Turn on certain internal resistance processes.
- Change to protect yourself from antibiotics.
- Receive resistant genes from other bacteria.
A. Antibiotic Resistance Increases When We Use Them
Antibiotic use is the major contributor to antibiotic resistance. When people take antibiotics, some bacteria are killed, but resistant bacteria can survive and even multiply. The overuse of antibiotics makes resistant bacteria more common. [4]
Bacteria have a higher chance of developing antibiotic resistance the more frequently we use antibiotics. As a result, antibiotics won't be beneficial in the future when we'll need them. If we decrease antibiotic use, the antibiotics may again become effective at killing bacteria. [4]
IV. NEED OF NEW ANTIBIOTICS
Modern medicine has undergone a revolution since the first antibiotic, penicillin, was discovered more than 90 years ago. Since then, antibiotics have emerged as one of the most widely used classes of medications. They are now used to both prevent and treat infections as well as to enable complex procedures that are now performed on a regular basis, such as organ transplants, caesarean sections, and hip replacement surgeries. [5]
Infections that are resistant to medication pose a severe hazard to public health today. Each year, illnesses that are resistant to current medications claim hundreds of thousands of lives. To save modern medicine, new antibiotics must be discovered that can eradicate bacteria that are resistant to existing medicines. [5]
But that’s only part of the solution, as over time bacteria will learn to resist the new drugs too. To stay ahead of the game in this constant race against superbugs, we also need innovations in developing vaccines and diagnostics, and better prevention control and surveillance. [5]
It is widely accepted that keeping pace with accelerating antibiotic resistance – three actions are needed:[6]
- Existing antibiotics must be kept effective including through infection prevention strategies and by optimizing their use through stewardship.
- Access to existing effective antibiotics must be expanded to everyone in need.
- The antibiotic pipeline must be filled and continually replenished to allow new effective types of antibiotics to be developed.
- 34 years have passed since the discovery of the last class of antibiotics. All antibiotics discovered since then have been modifications of existing classes, which means that resistance to them can develop more quickly. As drug resistance increases, the medical need for new drugs becomes evident. [6]

V. PREVENTIVE STRATEGIES AND MEASURES TO CURB ANTIBIOTIC RESISTANCE
Due to systematic abuse and excessive use of antibiotics in human medicine and food production, modern medicine is threatened by antibiotic resistance, which poses a threat to its effectiveness and, more importantly, the ability to respond to infectious diseases quickly and decisively on a global scale. In fact, drug-resistant microbes have arisen as a result of the excessive or improper use of such treatments in humans, animals, or crops. These organisms have evolved under strong selective pressure. The WHO decided to implement the Global Action Plan on Antimicrobial Resistance, which is based on five rigorous objectives, in 2015 after becoming aware of the enormous problem of antibiotic resistance: [2]
- To increase knowledge and comprehension of antibiotic resistance.
- To increase data volume and knowledge.
- To decrease the prevalence of illnesses by using efficient hygiene methods.
- To maximize the use of antibiotics for improving both human and animal health.
- To boost spending on novel medications, diagnostic equipment, vaccinations, and other therapies.
The fight against antibiotic resistance is given sufficient attention by organizations other than the WHO, such as the World Organization for Animal Health and the Food and Agriculture Organization of the United Nations. The use of antibiotics in veterinary medicine is of paramount importance. To stop the spread of illnesses caused by the huge number of animals maintained in confined spaces, it is required to strengthen the regulatory framework for medicated food and feed, which is primarily utilized in intensive farming. [2]To this goal, not only in human health but also in animal medicine, the surveillance and monitoring systems for resistant bacteria and the indiscriminate use of antibiotics have grown. In general, it is important to steer clear of reusing the same molecule and to encourage patient compliance with the proper timing and dosage of medications. [2]
Therefore, developing new compounds is essential. Additionally, existing antibiotics must be used more effectively, and research on increasingly reliable diagnostic tools for identifying antibiotic-resistant bacteria and evaluating antibiotic sensitivity must be encouraged. [2]
VI. SOME EXAMPLES OF NEW ANTIBIOTICS
A. Plazomicin
As with other synthetic aminoglycosides, plazomicin inhibits the production of bacterial proteins and exhibits in vitro dose-dependent bactericidal action. Plazomicin (marketed under the name Zemdri) was given FDA approval in 2018 for treatment in cUTI and pyelonephritis at a dose of 15 mg/kg IV, QD. Nephrotoxicity and ototoxicity are potential adverse reactions listed in the plazomicin FDA package insert. Plazomicin's nephrotoxicity in patients was comparable to that of other medications, according to a pooled review of three studies on the medicine. [7]
B. Eravacycline
Eravacycline is a fluorocycline of the tetracycline class. It prevents bacterial protein production just like other tetracyclines do. Eravacycline (trade name Xerava) was approved by the FDA in 2018 for the treatment of cIAI at a dose of 1 mg/kg IV, BD for a total of 4 to 14 days. Similar side effects to other tetracyclines, like hypersensitivity responses and long-lasting tooth discoloration, could occur with this medication. Further, the most common adverse events are infusion-site reactions, nausea, vomiting, and diarrhea. [7]
C. Temocilin
Ticarcillin, a penicillin antibiotic that primarily targets PBP3, was created and marketed in the UK in the 1980s but was quickly discontinued due to its ineffectiveness against Gram-positive bacteria, nonfermenters (such as A. baumannii and P. aeruginosa), and anaerobes. Temocillin is a derivative of ticarcillin. However, given the increasing incidence of third-generation cephalosporin-resistant Enterobacteriaceae infections, there has been renewed interest in this antibiotic as an alternative to carbapenems over the past decade. [7]
D. Ceftazidime/Avibactam
The FDA approved ceftazidime-avibactam (trade name Avycaz) for the treatment of cIAI (in combination with metronidazole) and cUTI in 2015 at a dose of 2.5 g IV, TD, and expanded it to HAP/ VAP in 2018. According to the FDA - Potential side effects of ceftazidime/avibactam were comparable to those of ceftazidime alone; the most commonly reported adverse reactions (in ≥5% of patients) were nausea and diarrhea, and a positive direct Coombs test. [7]
E. Ceftolozane/Tazobactam
Ceftolozane/tazobactam is an antipseudomonal cephalosporin and BLI tazobactam combination. The FDA granted approval for this antibiotic combination (sold under the brand name Zerbaxa) in 2014 for the treatment of cUTI and cIAI at a dose of 1.5 g IV, TD. A HAP/VAP indication was added in 2019. According to the FDA package insert, the two potential side effects that were most frequently reported (in 5% of patients) with ceftazidime/avibactam were nausea and diarrhea. [7]
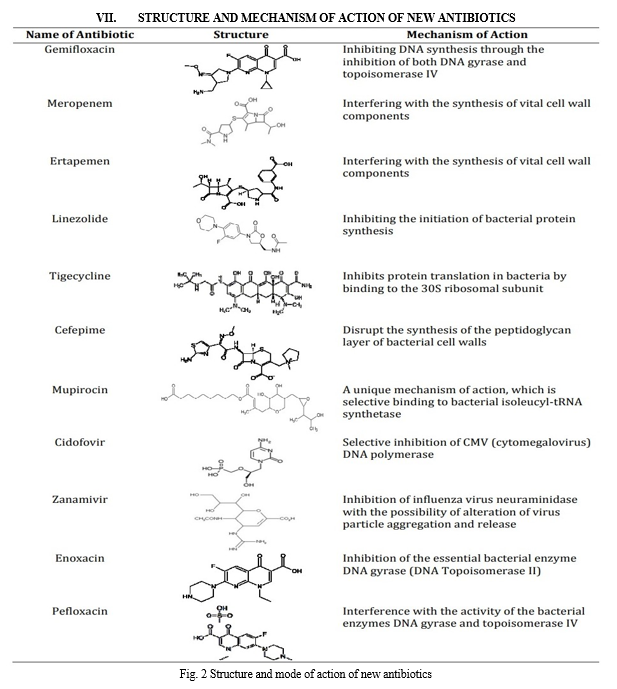
VIII. BENEFITS OF NEW ANTIBIOTICS
- Suitable for uncontrolled infection.
- Broad spectrum activity
- Greater bactericidal effects.
- Better oral compliance.
Conclusion
There aren\'t enough medications being developed right now to combat the growing issue of antibiotic resistance. The Pew Charitable Trust and the World Health Organization estimate that there are 40 to 50 antibiotics now in clinical research. In comparison to currently available treatments, many of these will only offer modest advantages. Only a small number of antibiotics specifically target Gram-negative bacteria, the most hazardous resistant bacteria that can result in life-threatening illnesses such as meningitis, pneumonia, and bloodstream infections. [5] In order to guarantee a sustainable pipeline of new medications, businesses, governments, and charitable organizations must collaborate. There are various choices. The world needs to act rapidly to safeguard modern medicine from the growing danger of infections with medication resistance while there is still time. [5] It\'s crucial to understand the proper way to take antibiotics. To find out how many tablets and how frequently to take your medication, read the package. Ask your pharmacist if there is anything else you need to be aware of regarding the drug. [1] We should take our entire course of antibiotics. Even though we feel better before our medicine is entirely gone, this is important for our healing. If the antibiotics are stopped halfway through treatment, the bacteria are only partially treated but not completely killed, leading to the development of antibiotic resistance. This causes a serious problem if those now-resistant bacteria grow enough to cause a re-infect. [1]
References
[1] R. N. Sharma and M. T. Patel, \"NEW ANTIBIOTICS: A REVIEW AND SURVEY OF CENTRAL GUJARAT IN INDIA,\" International Journal of Innovations in Pharmaceutical Science, vol. 2(4), no. 20-27, pp. 1,2,5,8, 02 June 2014. [2] M. Terreni, M. Taccani and M. Pregnolato, \"New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives,\" molecules, vol. 26, no. 2671, pp. 1,2,3, 02 May 2021. [3] M. Miethke, M. Pieroni, T. Weber, M. Brönstrup, P. Hammann, L. Halby, P. B. Arimondo, P. Glaser, B. Aigle, H. B. Bode, R. Moreira, Y. Li, A. Luzhetskyy, M. H. Medema, J.-L. Pernodet, M. Stadler, J. R. Tormo, O. Genilloud, A. W. Truman, K. J. Weissman, E. Takano, S. Sabatini, E. Stegmann, H. Brötz-Oesterhelt, W. Wohlleben, M. Seemann, M. Empting, A. K. H. Hirsch, B. Loretz, C. M. Lehr, A. Titz, J. Herrmann, T. Jaeger, S. Alt, T. Hesterkamp, M. Winterhalter, A. Schiefer, K. Pfarr, A. Hoerauf, H. Graz, M. Graz, M. Lindvall, S. Ramurthy, A. Karlén, M. v. Dongen, H. Petkovic, A. Keller, F. Peyrane, S. Donadio, L. Fraisse, L. J. V. Piddock, I. H. Gilbert, H. E. Moser and R. Müller, \"Towards the sustainable discovery and development of new antibiotics,\" Nature Reviews Chemistry, vol. 5, no. 726–749, p. 2, 19 August 2021. [4] \"What causes AMR?,\" 31 October 2022. [Online]. Available: https://www.amr.gov.au/about-amr/what-causes-amr. [Accessed 18 January 2023]. [5] \"Why is it so hard to develop new antibiotics?,\" 21 January 2020. [Online]. Available: https://wellcome.org/news/why-is-it-so-hard-develop-new-antibiotics. [Accessed 18 January 2023]. [6] \"The world needs new antibiotics – so why aren’t they developed?,\" SIDA, Uppsala University and others, 2021 October 2021. [Online]. Available: https://www.reactgroup.org/news-and-views/news-and-opinions/year-2021/the-world-needs-new-antibiotics-so-why-arent-they-developed/. [Accessed 18 January 2023]. [7] Y. Erlangga, H. I. Bax, N. J. Verkaik and M. v. Westreenen, \"An Update on Eight “New” Antibiotics against Multidrug-Resistant Gram-Negative Bacteria,\" Journal of Clinical Medicine, vol. 10(5), no. 1068, pp. 2-8, 04 March 2021.
Copyright
Copyright © 2023 Aarti S. Khobragade, Ajay P. Patil, Shubham B. Yadav, Dr. Rupali Tasgaonkar, Vishal T. Khandebarad. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET48972
Publish Date : 2023-02-02
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

