Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Comprehensive Review on Silymarine for the treatment of Atopic dermatitis
Authors: Parag Sharma, Prabal Bratt Sharma, Akshay Saxena, Priya Kumari, Ritik Sharma, Pravin Kumar, Mahendra Singh Ashawat
DOI Link: https://doi.org/10.22214/ijraset.2023.54641
Certificate: View Certificate
Abstract
Atopic dermatitis is a chronic inflammatory disease that causes eczema, rashes, and itching. It is most common in younger people & adolescence. Recurrent relapses are a characteristic feature of atopic eczema. Anti-inflammatory therapy of exacerbations is aimed to control effectively disease activity and permit a return to basic dermatological therapy as soon as possible. Oxidative stress & inflammatory responses are thought to be responsible for the pathogenesis of atopic dermatitis. Silymarin is a polyphenolic flavonoid derived from the seed of milk thistle (Silybum marianum (L.) Gaertner) that has anti-inflammatory, antioxidant and cytoprotective effect. Silymarin is a naturally occurring flavonoid medication, and recent studies have shown that it may be used to treat atopic dermatitis (AD). The primary goal of this effort is to formulate silymarin into a pluronic-lecithin organogel (PLO) base for topical skin administration. Utilizing two cosolvent systems of ethyl alcohol and dimethyl sulfoxide, six distinct PLO formulations with varied pluronic to lecithin ratios were created.
Introduction
I. INTRODUCTION
Atopic dermatitis (AD) is a continuously degeneration inflammatory illness that causes extremely pruritic skin and is common in youngsters[1].This is linked to increased IgE production and/or changed pharmacological responsiveness[2].In untreated individuals, the typical signs of AD with skin dryness, erythema, and oozing, which is sometimes followed by smearing and lichenification of the skin. Pruritus and skin itching and rashing are the bulk bothersome symptoms, accounting for a large portion of the illness loadfor patients and their families[3].As a result, the primary objective of managing AD patients is to employ topical anti-inflammatory medicines in conjunction with optimal skin hydration. In extreme circumstances, some individuals may require phototherapy or systemic treatment[4][5].Occupational contact dermatitis is a widespread health issue in the workplace and one of the main occupational ailments in many countries[6][7].Irritant contact dermatitis (ICD) is a localized, non-allergic inflammatory skin response that generally shows by Both acute and long-term exposure to a chemical or physical irritant can result in edema and erythema. According to reports, ICD accounts for 70–80% of occupational contact dermatitis[7].It is commonly known that people who stop using oral steroids relapse. Using corticosteroids long-term is linked to a number of known adverse effects, including: greater risk of cataracts and glaucoma[8].In addition to the increased rate of steroid addiction after cessation[9].In recent years, there has been an increase in interest in employing herbal remedies to treat skin conditions[10-13].A naturally occurring polyphenolic flavonoid called silymarin is produced from milk thistle seeds. It has a lengthy history of usage as a conventional herbal hepatoprotectant[14].According to Zhao et al., silymarin has a variety of effects on the skin, including chemoprotective and anticancer qualities[15].Additionally, silymarin was discovered to have a skin-whitening impact and play a part in the protection of UV-B-induced skin damage[16,17].Additionally, silymarin's anti-inflammatory and antioxidant properties have a favorable impact on skin burns and the healing of wounds[18-20].Silymarin is a standardized seed extract that is high in flavonolignans, a class of flavonoid chemicals. The isomers of silybin (also known as silibinin), silydianin, and silychristin are the primary flavonolignans in silymarin. The flavonoid taxifolin reacts oxidatively with coniferyl alcohol to produce silybin.
The most significant component of silymarin, silybin, has been the focus of several biochemical and pharmacological investigations. The silymarin extract also contains dehydrodiconiferyl alcohol, quercetin, taxifolin, and silybin oligomers, among other substances[21].The silymarin flavonolignans are rapidly but insufficiently absorbed from the digestive system.The bioavailability of silymarin in humans appears to be poor, like that of many other phenolic compounds.Silybin's plasma concentrations peak 1.3 hours after oral administration, and its elimination half-life is roughly 6 hours.
Bile excretion appears to be the primary method of elimination, with urine excretion playing a smaller part.At least in part, silybin and silychristin are eliminated in the bile as glucuronides and sulfates.The silymarin's high level of liver specificity may be attributable to the components' enterohepatic circulation, or their excretion with bile into the digestive system where they can be reabsorbed[22].
II. MATERIALS AND METHODS
- Subjects: Atopic dermatitis does not have a single clinical symptom or a biological change that is constantly seen. The most well-known diagnostic criteria were proposed by Hanifin and Rajka, and as a result, the diagnosis of atopic dermatitis is predicated on the existence of these criteria in the modern day[23].The trial included patients with moderate to severe atopic dermatitis. According to accepted standards, patients had been diagnosed with confirmed atopic dermatitis. The following conditions were excluded: clinically significant heart disease, other severe concurrent illnesses, an active infection, pregnancy or breastfeeding, and CNS or seizure problem[24].
- Materials: White petrolatum was used to manufacture Silymarin, which was produced by the German company Luna Co. and sold as an ointment for topical application in concentrations of 0.05%, 0.1%, and 0.2%, in SDI, Iraq.
- Study Design: For eight weeks, 43 patients received topical preparations of silymarin at concentrations of 0.05%, 0.1%, and 0.2% twice daily. A single-blind, placebo-controlled trial with forty individuals was conducted (three patients dropped out).Patients were checked on for security, effectiveness, healing, and progress every three days. assessments that take into account the overall body surface area involved and clinical severity score. Six characteristics made up the total clinical severity score, each of which was scored on a half-point scale: erythema, edema/papulation/induration, pruritus, excoriations/erosions, scaling/dryness, and lichenification incremental scale with 1 being mild, 2 being moderate, and 3 being severe.
- Statistical Analysis: Analysis of the quantity and percentage of age groups, the therapy, and the improvement within participants. The data were compared using a paired t test (P 0.05).Chi-square technique to identify areas of growth (P 0.05).The linear relationship between the total body surface area involved and the overall clinical severity score at baseline and the change in these measures throughout the course of the trial were evaluated using Pearson correlation (r).
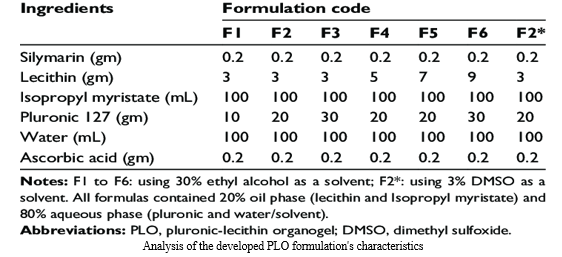
- Preparation of silymarin PLO gels: The composition of the various PLO formulations is shown in Table 1 following the preparation of various PLO formulations. By allowing its components to dissolve in the right amount of IPM for the duration of the night at room temperature, the oil phase, which contains soy lecithin, was created. To create the aqueous phase, the measured amounts of pluronic F-127 and ascorbic acid were dissolved in cold water and stored in a refrigerator (at 4°C).In either ethyl alcohol or DMSO, silymarin was dissolved, then combined with the ready aqueous phase. During a minute-long procedure at room temperature, the aqueous phase (80%) was gradually introduced drop-by-drop to the oil phase (20%) while being continuously stirred (at 1,000 rpm). An electronic pH meter (produced by HANNA Instruments, Woonsocket, Rhode Island, USA) was used to measure the pH of the final organogel formulations.
6. Fourier Transform Infrared Spectroscopy (FTIR): Silymarin, unloaded PLO gel, and plain PLO gel samples (formulation 2 "F2"), each weighing 1 mg, were dried, mixed with potassium bromide, compressed into discs at a pressure of 6 ton/cm2, and then scanned using a Fourier transform infrared spectrophotometer (PerkinElmer Inc., Waltham, MA, USA). The benchmark was an empty KBr pellet.
7. Drug Content: One gram of each formulation of the manufactured PLO gels was put into a 50 mL volumetric flask in order to determine the amount of drug present. To dissolve the medication, the volume was made up with ethyl alcohol and agitated for 10 minutes at 100 rpm. Filters with a 0.22 m pore size were used to filter the samples. One milliliter of the filtrate was taken out and diluted with ethyl alcohol to make 10 milliliters in a volumetric flask. With the use of a spectrophotometer (SpectronicGenesys®, with Winspec Software; Spectronic 700, NY, USA), the drug's concentration was calculated spectrophotometrically at a wavelength of around 286 nm.
8. In Vitro Release Studies: Silymarin PLO formulations' in vitro release was investigated utilizing a phosphate buffer with a pH of 7.4 and 20% ethyl alcohol as the release medium. The discharge research was carried out in a sink environment. A cellophane membrane with a molecular weight cutoff of 6,000–8,000 Da was placed over one end of a cylindrical glass tube with a surface area of 2.8 cm2 and presoaked in the releasing medium. The inside of the cellophane membrane was coated with one gramme of the formulation under examination, and the complete assembly was immersed in 100 mL of the release media at 37°C0.5°C and violently shaken 100 times per minute. The predetermined intervals were used to collect 5 mL samples. Each sample was replaced with an equal volume.
9. Ex Vivo Permeability Study: Ex vivo permeability tests were performed on selected formulations (F2 and *F2) using excised rabbit skin. The test was conducted as described in the section under "In vitro diffusion study," with the exception of utilizing rabbit ear skin that had been removed. The removed skin was properly cleansed by being thoroughly washed under cold running water. Any remaining fatty material was then treated by immersing the entire skin membrane in water heated to 60 °C for 70 seconds, followed by blunt dissection, before being folded in aluminum foil. The epidermal side of the mounted epidermal membranes was towards the donor solution after they had been prepared.
Conclusion
Silymarin has the capacity to prevent the production of proinflammatory leukotrienes by inhibiting the enzyme 5-lipoxygenase. Leukotriene receptor antagonists are a novel therapy strategy for AD , silymarin is a novel medicine for this strategy, and our study supports the preceding findings. Silymarin has considerably decreased histamine production, which accounts for the relief of intense itching and red, swollen skin that is associated with eczema at all doses employed for topical therapy in this study. According to a theory, silymarin works by altering how mitogen-activated protein kinase and other cellular cycle regulators are active. Recent research shown that silymarin prevented the activation of NF-B and apoptosis caused by TNF.PLO gel with silymarin was successfully made. The indications and symptoms of AD patients significantly improved because to the base\'s great penetrating ability and hydration effect. So it\'s possible to draw the conclusion that silymarin loaded PLO gel might be launched as a unique topical silymarin formulation. Atopic dermatitis can be managed more effectively, safely, and effectively with topical silymarin therapy.
References
[1] Darsow U, Raap U, Ständer SR. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton: CRC Press; 2014:832–836. [2] Ring J, Przybilla B, Ruzicka T. Handbook of atopic eczema. 2nd ed. New York: Springer-Verlag Berlin Heidelberg; 2006. [3] Friedmman PS, Holden CA. Atopic dermatitis. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s Textbook of Dermatology. 7th ed. Oxford, England: Blackwell Sciences; 2008:755–786. [4] Charman C. Clinical evidence: atopic eczema. BMJ. 1999;318(7198):1600–1604. [5] Sudilovsky A, Muir JG, Bocobo FC. A comparison of single and multiple applications of halcinonide cream. Int J Dermatol. 1981;20(9):609–613. [6] Saary J, Qureshi R, Palda V, DeKoven J, Pratt M, Skotnicki-GrantS, et al. A systematic review of contact dermatitis treatmentand prevention. J Am Acad Dermatol 2005;53:845–55. [7] Nixon R, Frowen K, Moyle M. Occupational dermatoses. AustFam Physician 2005;34:327–33. [8] Haeck IM, Rouwen TJ, Mik LT, Bruin-Weller MS, Bruijnzeel-KoomenCA.Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64(2):275–281 [9] Hajar T, Leshem YA, Hanifin JM, et al. A systematic review of topical corticosteroid withdrawal (‘‘steroid addiction’’) in patients with atopic der¬matitis and other dermatoses. J Am Acad Dermatol. 2015;72(3):541e–549e.e2. [10] O’Hara M, Kiefer D, Farrell K, Kemper K. A review of 12 commonly used medicinal herbs. Arch Fam Med. 1998;7(6):523–536. [11] Zhao J, Lahiri-Chatterjee M, Sharma Y, Agarwal R. Inhibitory effect of a flavonoid antioxidant silymarin on benzoyl peroxide-induced tumor promotion, oxidative stress and inflammatory responses in SENCAR mouse skin. Carcinogenesis. 2000;21(4):811–816. [12] Zhao J, Lahiri-Chatterjee M, Sharma Y, Agarwal R. Inhibitory effect of a flavonoid antioxidant silymarin on benzoyl peroxide-induced tumor promotion, oxidative stress and inflammatory responses in SENCAR mouse skin. Carcinogenesis. 2000;21(4):811–816. [13] Singh RP, Agarwal R. Cosmeceuticals and Silibinin. Clin Dermatol. 2009;27(5):479– 484. [14] Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell func¬tions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23(4):749–754. [15] Zhao J, Lahiri-Chatterjee M, Sharma Y, Agarwal R. Inhibitory effect of a flavonoid antioxidant silymarin on benzoyl peroxide-induced tumor promotion, oxidative stress and inflammatory responses in SENCAR mouse skin. Carcinogenesis. 2000;21(4):811–816. [16] Gazak R, Walterova D, Kren V. Silybin and silymarin – new and emerg¬ing applications in medicine. Curr Med Chem. 2007;14(3):315–338. [17] Rasul A, Naveed A, Ali A, et al. Assessment of anti erythmic and skin whitening effects of milk thistle extract. African Journal of Pharmacy and Pharmacology. 2011;520(20):2306–2309. [18] Evans WC. Trease and evans Pharmacognosy. London: WB Saunders company Ltd, 1996. [19] Wijesekera ROB. The Medicinal Plant Industry. Boca Raton, Florida: CRC Press, 1991. [20] Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta DermVenereol 1980; 92: 44-7. [21] Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta DermVenereol 1980; 92: 44-7.
Copyright
Copyright © 2023 Parag Sharma, Prabal Bratt Sharma, Akshay Saxena, Priya Kumari, Ritik Sharma, Pravin Kumar, Mahendra Singh Ashawat. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET54641
Publish Date : 2023-07-06
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

