Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Evaluation of Soils Affected by Salts in the Al-Jufra - Sokna Area
Authors: Hanan Mohamed Nasr, Abdulsalam Salem Mohamed
DOI Link: https://doi.org/10.22214/ijraset.2024.64955
Certificate: View Certificate
Abstract
This study was conducted to assess the extent of the impact of natural, environmental and human factors on soil salinity and its distribution variation in the Al-Jufra region, specifically in the Al-Hamam Agricultural Project - Sokna, in addition to identifying the type of soil prevailing in the region. To achieve the objectives of this study, a series of laboratory experiments were conducted where samples were collected from the surface layer 0 - 30 cm and the subsurface layer 30 - 60 cm and were prepared and some physical and chemical properties were estimated, including pH, EC, CaCO3, O.M. CEC. After conducting the tests, it was noted that most of the soils in the study area have three different types of texture. The results of the chemical analysis showed a high electrical conductivity of the surface layer soil compared to the subsurface layer. On the other hand, the results showed high ESP values for most soils, as its value in the surface layer was higher than the lower layer of the soil. The study shows the role played by human factors in the increasing phenomenon of salinization of agricultural soils in the project area through mismanagement by leaving the land fallow after exploitation, and the lack of drains and the low efficiency of the existing ones.
Introduction
I. INTRODUCTION
Soil salinity is one of the most important and influential challenges for the agriculture and environmental development sectors due to its negative impact on food security and the environment. Soil salinity and the scarcity of usable water resources are the biggest challenges and concerns of humanity since the beginning of the twenty-first century.
The problem of salt-affected soils is a reality in the lands of arid and semi-arid regions, estimated at about 2.46 million hectares in Libya, and most of these lands are concentrated in the southern regions where there is little rainfall and high rates of evaporation. The area of lands affected by salinity is increasing at a rate of 2 million hectares annually as a result of climate change and continued irrigation with low-quality water, in addition to high rates of evaporation and weathering of rocks. It is predicted that 50% of the lands not reclaimed for agriculture may suffer from this problem by the year. About 20% of the world's irrigated lands are affected by high soil salinity, and this rate rises to 30% in the dry and semi-dry regions of the world [1].
The classification of soil salinity and the establishment of data on the management and quality of soils affected by salts, and soil salinization transforms vast areas of arable and productive lands into completely or partially unproductive lands due to the accumulation of salts, which are a limiting factor for plant growth.
Salinization is considered one of the main causes of soil degradation, as it leads to a change in the physical and chemical properties of the soil. Saline soils contain soluble salts in sufficient quantities to obstruct plant growth. Sodium, for example, affects the properties of the soil, as the soil may become sodic and low in permeability [2], this is because the soil constitutes a very important part of renewable natural resources, and a basic element in agricultural production.
The increase in population and the irrational management in the study area also play an important role, such as trying to increase production through intensive agriculture, multiple plantings, and excessive use of fertilizers without using the correct scientific methods, and irrigation with low-quality water, which led to soil degradation as an irreplaceable source of production and is still being misused. Because the Sokna area is one of the areas where areas of land affected by salts are spread, and because of the importance of this phenomenon, which is considered one of the reasons that led to soil degradation and its transformation into an unsuitable environment for the growth of some plants and crops, to identify the effects of this phenomenon in the region and search for its causes, evaluate them, and search for appropriate solutions, the study aimed to:
- Study some of the natural and chemical properties of the soil.
- Classify the soil in the study area.
- Alert and warn decision-makers of the existence of areas threatened by soil degradation.
II. PREVIOUS STUDIES
Soil is a system consisting of three phases. The solid phase is considered a cohesive mixture of mineral and organic particles, so that the mineral part is responsible for determining the general properties of the soil, which are related to the proportions of sand, silt and clay. The mineral part is relatively stable, so the properties resulting from it can be considered permanent aspects of the soil. The physical properties of the soil contribute to obtaining a basic concept of the mechanical system controlling soil behavior and its role in the biosphere. It also contributes to the process of appropriate soil management through irrigation, drainage, maintenance, tillage, aeration and regulation of soil temperature, as the properties of the soil depend in general on the nature of its components [13]. The arrangement of the solid components of the soil is what determines the properties of the spaces and porosity in which water and air move and are held. Common physical properties in most dryland soils include the ability to form surface crusts [43], impermeability and high susceptibility to erosion [20]. Low water retention capacity and soil profile stiffness [44].
Brady (1974) explained that the personal properties are present on the spread and productivity of plants according to their tolerance to salinity. Agricultural soils differ in their content of the remaining biological matter, after remaining and the microorganisms that have not yet completed their effectiveness in affecting the physical and biological crops of the soil [45], if some of it decreases, but then the membership returns to the harmful effect of salts in the soil on growth from now on and quality, as the levels of plant humus in soils affected by salts decrease to low levels which helps with what is involved in it [35].
Calcium carbonate is one of the most common salts in the soils of arid and semi-arid regions, as its formation in the soil depends on temperature, pH, and the activity of calcium ions in the soil solution, as well as on carbon dioxide gas in the soil, in addition to high evaporation [36]. Inorganic carbonates are present in the soil in the form of calcium carbonate (calcite), or magnesium carbonate (dolomite), or a mixture of both, as a result of weather factors or because they are inherited from the parent material [38]. When there is a high percentage of calcium carbonate in one of the soil horizons at a higher percentage than the horizon following it, it is called (calcareous horizon) [29]. The presence of this horizon contributes to the formation of a salt crust, which leads to soil degradation and hinders the natural growth of plants [31].
Climatic, hydrological, topographical and other factors cause the salinization phenomenon, as ions begin to combine to form salts that cause soil salinization, and these factors increase its severity [11]. While Al-Mahi et al (1996) indicated that improper management of soil and water is the most important cause of salinization.
Saline soils are characterized by the electrical conductivity of the saturated paste extract of the soil sample at saturation being more than 4 dS.m-1 at a temperature of 25°C, and the percentage of exchangeable sodium being less than 15% of the cation exchange capacity, and its pH being less than 8.5. As a result of the low exchangeable sodium, the soil particles are compact and well permeable, similar to non-saline soils, but their structure is unstable and in most cases there is a crust of crystalline salts on their surface, which increases in the surface layers and decreases in the lower layers before washing and cultivating them [46].
III. DESCRIPTION OF THE STUDY AREA
The Al-Jufra region is located between the Sirte Basin (east) and the Al-Hamada Al-Hamra (west). The ground level ranges between 290 - 350 meters above sea level and reaches more than 500 meters above sea level on the southern and western edges. The Al-Jufra region is generally characterized by a hot, dry desert climate in summer and cold in winter. Summer is the longest period extending from the beginning of April to the end of September, interspersed with some sandstorms, especially in April. Temperatures in the summer range between 35 - 40 degrees Celsius and sometimes more. The winter season is characterized by severe cold, especially at night, where temperatures reach zero, while the annual rainfall does not exceed 30 mm / year.
The study area is located in the central eastern edge of the northwestern part of Libya between longitudes 50 - 150' 35' 150 east and latitudes 280 55' - 290 10' north. The area of the study area (Al-Hamam Agricultural Project) is about 1100 hectares.
IV. GEOLOGY OF THE AREA
The study area shows a clear diversity in the discovered formations. In the Sukna area and its surroundings, Paleocene and Upper Cretaceous (Thanitic) rocks appear with the presence of Quaternary sediments represented by aeolian sediments and fan sediments (gravel and boulders with sand and sandy clay), especially in the valleys on the western side of Sukna. The area is also affected by many structural elements that have an impact on the stratigraphic sequence of the various rock formations, their extension and depths. Also, the Hun Rift is considered the western end of the Sirte Basin and is a major structural element that separates the geological and hydrological situation between the Sukna area, which is considered outside the scope of the rift, and the Waddan and Hun areas, which are located within it [3].
V. FIELD WORK
Thirty soil samples were collected for the site after conducting a field visit to the site and identifying the most important morphological properties, identifying the terrain and determining the features of the soil surface, so that the site coordinates were recorded using the GPS device, Figure 1.
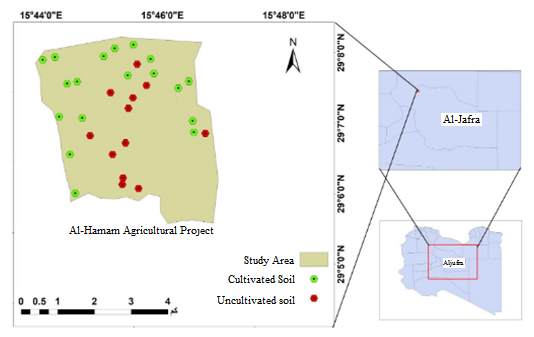
Fig 1: Site map of the study area and sample distribution
The selected soil samples were collected using the Auger device from depths of 0-30 cm and 30-60 cm according to what was stated as mentioned by Page in 1982, in addition to the crust layer formed on the surface in the areas wherever it was found, as the samples included cultivated and uncultivated lands (poor land), and the water content was measured at the time of collecting the samples. The soil was air-dried in the laboratory and sieved through a sieve with holes diameter of 2 mm and stored until the analysis was conducted.
VI. RESULTS
A. Soil Texture
The results of the mechanical analysis of the soil Table 1 showed that most of the soil samples under study were of sandy, sandy-silty or sandy-silty texture, and are classified as coarse textured soils. This type of soil is characterized by large pores and low water holding capacity, and tends to be loose and have good drainage and ventilation.
It found that the lower soil samples contained higher amounts of silt and clay than the surface layer samples and that the percentage of gravel in the studied soil samples, where the highest value was recorded for sample 7 while the lowest value was for sample 30, which is consistent with what was indicated by Claridge and Campbell (1982) that the soils of desert areas often have a sandy surface soil texture while the lower soil contains significant amounts of silt and clay. The soil texture of area No. 19 is sandy clayey, and this type of soil is characterized by containing small pores and is characterized by the presence of a solid layer at a depth of 40 cm for sample No. 19, 25 and 30 cm due to the compression and cohesion of the lower layers, which is consistent with what was stated by Atinson and Wangh, (1979) who indicated the presence of the impermeable layer in dry desert soils.
TABLE I
results of mechanical analysis of soils in the study area
|
Sample No |
Planted |
Depth cm |
Gravel % |
Sand % |
Silt % |
Clay % |
Description |
|
|
Not |
0-25 |
15.9 |
80.5 |
1.625 |
17.877 |
Sandy – Loam |
|
|
Not |
0-30 |
22.12 |
70.892 |
13.101 |
16.013 |
Sandy – Loam |
|
|
Yes |
0-30 |
18.62 |
77.168 |
8.124 |
14.708 |
Sandy – Loam |
|
-3060 |
0 |
73.940 |
8.200 |
17.860 |
Sandy – Loam |
||
|
|
Yes |
0-30 |
36.8 |
79.240 |
5.129 |
15.390 |
Sandy – Loam |
|
30-50 |
0 |
79.240 |
5.148 |
15.55 |
Sandy – Loam |
||
|
|
Yes |
0-30 |
41.35 |
82.899 |
3.115 |
14.018 |
Sandy – Loam |
|
-3060 |
0 |
87.984 |
4.333 |
13.481 |
Sandy – Loam |
||
|
|
Not |
0-30 |
25.47 |
73.935 |
1.684 |
10.332 |
Sandy |
|
-3060 |
0 |
87.984 |
4.333 |
13.481 |
Sandy – Loam |
||
|
|
Not |
0-30 |
51.08 |
84.484 |
3.103 |
12.413 |
Sandy – Loam |
|
-3060 |
0 |
52.030 |
32.523 |
15.462 |
Loamy – Sand |
||
|
|
Yes |
0-30 |
19.76 |
80.35 |
6.53 |
13.08 |
Sandy – Loam |
|
-3060 |
0 |
51.513 |
21.338 |
27.149 |
Sandy Clay Loam |
||
|
|
Yes |
0-30 |
2.8 |
87.891 |
2.987 |
9.122 |
Sandy |
|
-3060 |
0 |
85.512 |
3.663 |
19.825 |
Sandy |
||
|
|
Yes |
0-30 |
7.6 |
87.190 |
3.100 |
9.709 |
Sandy |
|
-3060 |
0 |
85.476 |
3.65 |
10.874 |
Sandy |
||
|
|
Yes |
0-30 |
1.49 |
88.765 |
2.196 |
9.039 |
Sandy |
|
-3060 |
0 |
70.892 |
4.557 |
10.074 |
Sandy |
||
|
|
Yes |
0-30 |
3.78 |
88.357 |
2.978 |
8.935 |
Sandy |
|
-3060 |
0 |
81.904 |
8.650 |
9.441 |
Sandy |
||
|
|
Yes |
0-30 |
3.065 |
87.512 |
6.244 |
6.244 |
Sandy |
|
-3060 |
0 |
85.459 |
5.831 |
8.824 |
Sandy |
||
|
|
Not |
0-30 |
7.49 |
86.459 |
3.009 |
10.532 |
Sandy – Loam |
|
-3060 |
0 |
74.3 |
6.446 |
10.150 |
Sandy – Loam |
||
|
|
Yes |
0-30 |
3.59 |
85.5 |
5.72 |
8.97 |
Sandy – Loam |
|
-3060 |
0 |
83.5 |
8.65 |
7.94 |
Sandy – Loam |
||
|
|
Yes |
0-30 |
5.34 |
77.78 |
19.24 |
2.96 |
Sandy – Loam |
|
-3060 |
0 |
72.591 |
11.2 |
8.16 |
Sandy Clay Loam |
||
|
|
Yes |
0-30 |
7.54 |
77.17 |
8.13 |
14.17 |
Sandy – Loam |
|
-3060 |
0 |
74.5 |
9.17 |
17.9 |
Sandy Clay Loam |
||
|
|
Yes |
0-30 |
4.72 |
85.344 |
6.446 |
8.825 |
Sandy |
|
-3060 |
- |
83.404 |
2.934 |
10.466 |
Sandy |
||
|
|
Not |
0-30 |
32.09 |
72.224 |
2.934 |
24.842 |
Sandy Clay Loam |
|
-3060 |
0 |
68.51 |
9.745 |
24.842 |
Sandy Clay Loam |
||
|
|
Not |
0-30 |
7.92 |
79.762 |
3.680 |
16.559 |
Sandy – Loam |
|
-3060 |
0 |
72.591 |
5.913 |
21.496 |
Sandy – Loam |
||
|
|
Yes |
0-30 |
4.369 |
80.070 |
15.944 |
9.863 |
Sandy – Loam |
|
-3060 |
0 |
78.340 |
16.269 |
5.423 |
Sandy – Loam |
||
|
|
Yes |
0-30 |
5.14 |
73.381 |
19.948 |
6.671 |
Sandy – Loam |
|
-3060 |
0 |
71.581 |
18.945 |
9.474 |
Sandy – Loam |
||
|
|
Not |
0-30 |
18.19 |
83.351 |
5.006 |
11.680 |
Sandy – Loam |
|
-3060 |
0 |
79.306 |
4.139 |
16.555 |
Sandy – Loam |
||
|
|
Not |
0-30 |
12.58 |
86.406 |
4.119 |
9.475 |
Sandy |
|
-3060 |
0 |
83.351 |
6.772 |
13.544 |
Sandy Clay Loam |
||
|
|
Yes |
0-30 |
15.17 |
76.766 |
7.266 |
15.985 |
Sandy – Loam |
|
-3060 |
0 |
60.255 |
9.935 |
29.803 |
Sandy Clay Loam |
||
|
|
Not |
0-30 |
12.58 |
87.785 |
1.525 |
10.69 |
Sandy |
|
-3060 |
0 |
87.785 |
12.076 |
21.236 |
Sandy – Loam |
||
|
|
Yes |
0-30 |
0.648 |
89.960 |
4.0165 |
6.0245 |
Sandy |
|
-3060 |
0 |
88.902 |
3.182 |
7.953 |
Sandy |
||
|
|
Not |
0-30 |
6.65 |
89.205 |
3.182 |
7.953 |
Sandy |
|
-3060 |
0 |
88.844 |
4.461 |
6.693 |
Sandy |
||
|
|
Not |
0-30 |
13.04 |
86.562 |
1.462 |
11.976 |
Sandy – Loam |
|
|
Not |
0-30 |
0.045 |
86.96 |
2.897 |
10.143 |
Sandy – Loam |
|
-3060 |
0 |
84.489 |
3.429 |
12.072 |
Sandy – Loam |
The high percentage of fine sand and its dominance over sand grains at a depth of 40 cm for sample 19, where the percentage of fine sand was 47.280%, which is consistent with what was indicated by both Swindale (1982) and Vial and Wright (1965) where they mentioned the existence of a great diversity in the texture of soils in dry and semi-dry areas between sandy, silty sand and silty sandy clay soils and that these soils are characterized by a wide range in terms of soil texture and characteristics of their section. As the results of mechanical analysis showed that the lower soil samples contained higher quantities of silt and clay than the surface layer samples and that the percentage of gravel in the soil samples under study ranged between 0.045, 51.08% for samples No. 30, 7 respectively, and this is consistent with what was indicated by Claridge and Campbell (1982) that the soils of desert areas often have a sandy surface soil texture while the lower soil contains significant quantities of silt and clay, as The results of other soil samples in the study area showed that medium sand prevailed in most areas, while the proportion of coarse and fine sand varied from one area to another.
B. Colour
The results of the color analysis of soil samples Table 2 showed that the surface layer is often darker than the lower layer at a depth of 30-60 cm in color, which is consistent with what was indicated by Bin Mahmoud and Al-Jundil (1984) who found that the colors of the surface layer are often darker than the lower layer. In general, most of the soils under study are characterized by light colors. The results of the cultivated soil samples showed that the colors of the surface layer were similar in the dry state of the soil, as the colors of samples 5, 9, 10, 11, 12, 15, 17, 18, 22, 27 and 30 varied between Reddish Yellow and Yellow, as these colors are evidence of weathering processes and are produced by different minerals such as silica oxides and the low content of organic matter in these soils [11]. As for the wet state, their colors varied between Yellow, Yellowish Brown, Strong Yellow, Strong Brown the colors of the lower layer were Brown, Reddish Yellow, Yellow, and this may be due to the presence of iron oxides responsible for the appearance of yellow, brown, and red colors [12].
Soil sample No. 8 was distinguished by the dark gray color of the surface layer in the dry state, while in the wet state the color was Very Dark Gray, which may be due to the presence of manganese compounds responsible for the gray color in particular, while sample 25 was distinguished by the pink color in the dry state, and this The color is consistent with what was indicated by Al-Darbi (1995), who stated that this color is formed in dry areas due to some dissolved salts in the upper part of the soil that settle in depth to form a thick layer over time, while in the wet state the color was Strong Brown. As for the uncultivated soils, the results of the physical analysis of the color of most samples in the study area were 6, 7, 20, 23, 24, 26, 28, 29 and 30 between Dark Brown, Dark Yellow Brown, Light Brown, Yellow Red, and Strong Brown, in both dry and wet states.
As for the crust samples taken from the soil surface, the results of the physical analysis of the samples in the study area show a difference in the colors of the crust formed on the uncultivated soil. Samples 1, 14 and 26 were white due to the accumulation of salts. As for sample No. 2 and 20, the crust color was Pole Brown, Light Brownish Gray. The presence of the brown color is attributed to the presence of iron compounds within the salts that make up the crust. As for the rest of the samples, the crust colors were Pink, Pinkish White, and this is due to their containing iron and manganese compounds [14].
C. Water Content
The results of the water content analysis of the studied soils were between 2.00 and 36.50%. The lowest value was recorded for sample 7, while the highest value was recorded for sample 1. This is due to periodic changes in humidity according to seasonal climatic conditions or due to the irrigation factor [15]. The water content at the time of collecting the samples of the cultivated soils ranged between 5 and 22.50%. The highest value was recorded for sample 25, while the lowest value was for sample 27 in the surface layer, while it ranged between 5.75 and 25.40% for the depth of 30-60 cm. The reason for this is that the texture of the soil in the study is a rough texture with large pores, so it is characterized by rapid wetting, especially when these pores form a connected capillary system within the soil profile [16]. On the other hand, the results show that the reason for the high value of the water content of sample 25 is due to the fact that it is a medium-textured soil with groundwater at a depth of more than 60 cm, and the vegetation cover plays an important role in reducing water loss through evaporation [47]. As for the uncultivated soil, the water content value of the samples ranged between 2.00 and 36.50%, with the lowest value recorded for sample 7, while the highest value was for sample 1 for the surface layer. While the values for the lower layer of the soil ranged between 3.20 and 11.90%, with the lowest value recorded for sample 7 and the highest value for sample 14.
As the results of the study show a high water content in the uncultivated soil from which sample 14 was taken, with values of 11.60 and 11.90% for the surface and lower layers respectively, due to its location in a low-lying area exposed to leaching from neighboring areas as a result of unregulated irrigation.
TABLE III
Analysis of colour, water content and porosity of soils
|
Sample No |
Depth cm |
Colour |
Water content % |
Porosity % |
|
|
Dry |
Wit |
||||
|
|
crust |
White |
Pink |
- |
- |
|
0-25 |
Strong Brown |
Dark Brown |
36.5 |
38.10 |
|
|
|
crust |
Light Brownish Gray |
Dar Grayish Brown |
- |
- |
|
0-30 |
Pinkish Gray |
Dark Reddish Gray |
25.00 |
38.10 |
|
|
|
0-30 |
Strong Brown |
Dark Brown |
9.11 |
39.0 |
|
-3060 |
Reddish Yellow |
Strong Brown |
9.81 |
- |
|
|
|
0-30 |
Light Reddish Brown |
Reddish Brown |
9.70 |
40.00 |
|
30-50 |
Light Brown |
Strong Brown |
8.15 |
- |
|
|
|
0-30 |
Reddish Yellow |
Strong Brown |
10.50 |
41.20 |
|
-3060 |
Brown |
Strong Brown |
9.94 |
- |
|
|
|
0-30 |
Brown |
Dark Brown |
2.90 |
39.40 |
|
-3060 |
Light Reddish Brown |
Dark Reddish Brown |
2.99 |
_ |
|
|
|
0-30 |
Light Yellow Brown |
Dark Yellowish Brown |
2.00 |
40.00 |
|
-3060 |
Light Gray |
Grayish Brown |
3.20 |
_ |
|
|
|
0-30 |
Dark Gray |
Very Dark Gray |
21.00 |
40.00 |
|
-3060 |
Very Dark Grayish Brown |
Very Dark GRAY |
21.90 |
_ |
|
|
|
0-30 |
Reddish Yellow |
Strong Brown |
9.07 |
40.00 |
|
-3060 |
Reddish Yellow |
Strong Brown |
7.87 |
_ |
|
|
|
0-30 |
Yellow |
Yellow |
9.69 |
40.20 |
|
-3060 |
Yellow |
Brown Yellow |
7.97 |
_ |
|
|
|
0-30 |
Yellow |
Yellowish brown |
8.61 |
39.80 |
|
-3060 |
Yellow |
Brownish Yellow |
7.86 |
-- |
|
|
|
0-30 |
Reddish Yellow |
Strong brown |
9.03 |
40.30 |
|
-3060 |
Reddish Yellow |
Reddish Yellow |
8.03 |
-- |
|
|
|
crust |
White |
Pink |
- |
- |
|
0-30 |
Very Pole Brown |
Yellowish brown |
9.86 |
38.60 |
|
|
-3060 |
Reddish Yellow |
Reddish Yellow |
8.76 |
- |
|
|
|
crust |
White |
Pink |
- |
- |
|
0-30 |
Very Pole Brown |
Yellowish brown |
11.60 |
35.50 |
|
|
-3060 |
Reddish Yellow |
Reddish Yellow |
11.90 |
- |
|
|
|
0-30 |
Reddish Yellow |
Strong brown |
9.63 |
39.00 |
|
-3060 |
Reddish Yellow |
Strong brown |
7.97 |
- |
|
|
|
0-30 |
Very Pole Brown |
Yellowish brown |
9.03 |
42.40 |
|
-3060 |
Yellow |
Yellowish brown |
8.86 |
- |
|
|
|
0-30 |
Reddish Yellow |
Strong Yellow |
8.34 |
42.70 |
|
-3060 |
Yellow |
Dark Yellowish brown |
8.39 |
- |
|
|
|
0-30 |
Reddish Yellow |
Strong brown |
6.69 |
38.40 |
|
-3060 |
Reddish Yellow |
Reddish Yellow |
8.26 |
- |
|
|
|
0-30 |
Light Reddish Brown |
Reddish Brown |
10.00 |
51.90 |
|
-3040 |
Reddish Yellow |
Strong Brown |
11.90 |
- |
|
|
|
Crust |
Pale Brown |
Dark Brown |
- |
- |
|
0-30 |
Light Brown |
Brown |
2.91 |
40.10 |
|
|
-3060 |
Pinkish Gray |
Dark Gray |
9.20 |
- |
|
|
|
0-30 |
Light Reddish Brown |
Reddish Brown |
19.61 |
39.60 |
|
-3060 |
Pink |
Strong brown |
16.36 |
- |
|
|
|
0-30 |
Reddish Yellow |
Strong Yellow |
15.35 |
40.20 |
|
-3060 |
Reddish Yellow |
Strong Yellow |
11.5 |
- |
|
|
|
Crust |
Pinkish White |
Pale brown |
- |
- |
|
0-30 |
Yellowish Red |
Reddish Brown |
4.14 |
41.10 |
|
|
-3060 |
dark brown |
Very Dark Brown |
4.48 |
- |
|
|
|
Crust |
Pink |
Strong brown |
- |
- |
|
0-30 |
Light Brown |
Dark Brown |
4.41 |
39.70 |
|
|
-3060 |
Brown |
Dark Brown |
4.30 |
- |
|
|
|
0-30 |
Pink |
brown |
22.50 |
47.50 |
|
-3060 |
Light Gray |
Gray |
25.40 |
- |
|
|
|
Crust |
White |
Pale Brown |
- |
- |
|
0-30 |
Reddish Yellow |
Strong Brown |
8.41 |
42.50 |
|
|
-3060 |
Reddish Yellow |
Reddish Yellow |
9.49 |
- |
|
|
|
0-30 |
Reddish Yellow |
Strong Brown |
5.00 |
40.80 |
|
-3060 |
Reddish Yellow |
Brown |
5.75 |
- |
|
|
|
Crust |
Pink |
Strong Brown |
- |
- |
|
0-30 |
Reddish Yellow |
Strong Brown |
3.14 |
40.60 |
|
|
-3060 |
Light Brown |
Strong Brown |
4.91 |
- |
|
|
|
Crust |
Pink |
Strong Brown |
- |
- |
|
0-30 |
Reddish Yellow |
Strong Brown |
16.55 |
40.20 |
|
|
|
Crust |
Pink |
Strong Brown |
- |
- |
|
0-30 |
Reddish Yellow |
Strong Brown |
7.00 |
40.00 |
|
|
-3060 |
Reddish Yellow |
Strong Brown |
8.60 |
- |
|
D. Porosity
The results of the soil analysis showed that the total porosity values of the surface layer of the soil ranged between 35.50 and 51.90%, which is within the range indicated by Al-Darbi (1995), where he stated that the total porosity range for different types of soil ranges between (30-60%).
The results also showed that the porosity of cultivated soils is relatively higher than that of uncultivated soils. The percentage values of total porosity of cultivated soils ranged between 38.40 and 51.90%. The highest value was for sample 19, while the lowest value was recorded for sample 18.
The uncultivated soils ranged between 35.50 and 42.50%. The lowest value was recorded for sample 14, while the highest value was recorded for sample 26. This may be due to the absence of the role played by plant roots in improving soil porosity due to the growth and penetration of roots, in addition to the role of microorganisms and their secretions in cultivated soils, which is consistent with what was indicated by Giuidi (1980).
Ismail (1988) and Brady (1974) mentioned that the porosity in medium and fine-textured soils ranges between (40-60%). This is what was shown by the results of the physical analysis of 19 soil samples, where the highest porosity values were recorded at 51.90%, due to the texture of the sandy clay loam soil. While we find that the porosity of the rest of the soil samples under study ranged between 35.50 and 42.60%, due to the fact that they are coarse-textured soils, which is consistent with what was indicated by Giuidi (1980) and Nikos at el. (2003), who mentioned that the porosity in sandy soils ranges between (35-50%).
E. PH
Chemical analyses of soil samples showed that the pH value of the soil at depths of 0-30 and 30-60 cm for cultivated and uncultivated soils ranged from neutral to alkaline, with values ranging from 7.00 to 8.45. The highest value was recorded for sample 26, while the lowest value was recorded for sample 21. This is consistent with what was stated by Jawad and Tawfiq (2002) and Bilal (2006) that the pH of soils in arid and semi-arid areas is usually neutral to alkaline. The variation in pH values in these soils may be due to the difference in soil properties and ionic content, which may play an important role in controlling soil pH.
It is also clear that there are no significant differences in pH at depths of 0-30 and 30-60 cm in the study area, as there were no significant changes in pH between depths. These results are consistent with what was stated by Osman et al. (2003).
While the pH of the surface crust layer ranged between 7.03 for sample 24 and 9.20 for sample 26, i.e. they are values tending towards alkalinity and the reason for this is the dominance of some positive ions in the soil that have the ability to hydrolyze such as sodium, potassium, calcium and magnesium, or basic salts such as calcium and magnesium carbonates.
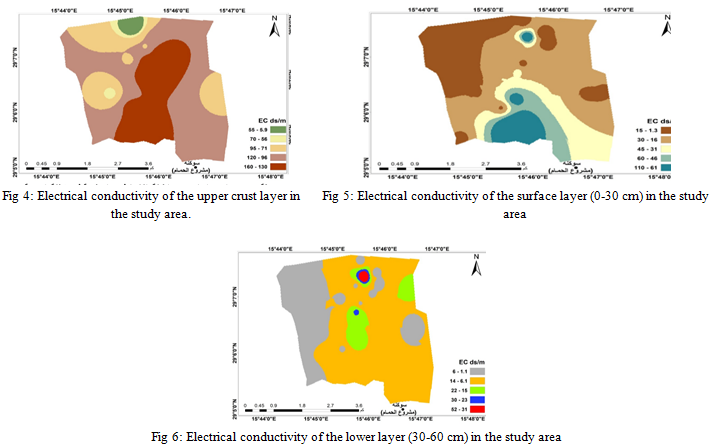
F. Electrical Conduction
The results of the chemical analysis of the study area showed differences in the electrical conductivity value of the soils under study, as we find that the electrical conductivity value of the soil extracts EC for the surface layer of cultivated soils ranged between 2.10, 30.04 dS.m-1, the highest value was recorded for sample 25, while the lowest value was recorded for sample 15, while the results for the extracts of the lower layer were between 1.03, 27.00 dS.m-1 for samples 9, 21 respectively.
As for the extracts of the surface layer of uncultivated soils, the values ranges between 10.31, 107.91 dS.m-1 and the lowest value was for sample 23, while the highest value was recorded for sample 2.
The values were between 3.69, 52.24 dS.m-1, where the lowest value was recorded for sample 24, while the highest value was recorded for sample 20, respectively for the extracts of the lower layer.
While it ranged between 5.86, 160.25 dS.m-1 for the crust layer extracts, the values were recorded for sample 17 as the lowest value and for samples 23, 30 as the highest value. It is clear from these results that the electrical conductivity values of the saturated extracts from the surface layer samples of the cultivated and uncultivated soil under study and the crust are higher than the lower layer; the reason for this is the upward movement of salts.
High evaporation levels indicate the accumulation of salts on the soil surface in the region, which is consistent with what was indicated by [24, 25, 26] that the electrical conductivity of the ground solution in soils affected by salts is higher in the surface layer of the soil and decreases with depth.
G. Positive Ions
1) Sodium
The results of the chemical analysis showed that the values of sodium ion concentration in the surface layer of the cultivated soil ranged between 8.50 and 181.51 milliequivalents/liter. The highest value was for sample 25, while the lowest value was recorded for sample 17.
A decrease in the concentration in the extracts of the lower layer soils, where the values ranged between 6.25 and 127.90 milliequivalents/liter for samples 9 and 21, respectively, while the results of the chemical analysis of the surface layer of the uncultivated soils ranged between 20.06 and 715.18 milliequivalents/liter. The lowest value was recorded for sample 26, while the highest value was for sample 2.
As for the extracts of the soil samples of the lower layer, its concentration ranged between 22.81 and 324.26 milliequivalents/liter for samples 24 and 20, respectively. The results of the chemical analysis show the dominance of sodium ions in the soils under study, which is consistent with what Ashraf (1999) indicated, as they all confirmed the dominance of sodium ions in soils in general due to its nature and that it is one of the basic ions that are easily weathered and highly soluble. The dominance of sodium ions in the soil solution is due to the accumulation of calcium and magnesium salts, which are mostly precipitated in the form of carbonates and sulphates, as indicated [28].
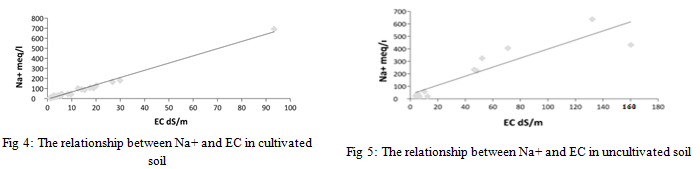
From the results of the statistical analysis, it was found that the concentration of sodium cation in cultivated and uncultivated soils had highly significant differences (p<0.05). There were also highly significant differences between the results obtained for the same sites for sodium cation (p>0.05) for the depths studied. There was also a very strong correlation between the concentration of sodium ions and the electrical conductivity values of the saturated soil paste extract for cultivated and uncultivated soil samples.
2) Potassium
The results of the chemical analysis of soil samples showed the presence of potassium in lower concentrations than calcium and magnesium because it is absorbed in larger quantities by plants. The value of potassium in the surface layer of cultivated soil ranged between 0.42 and 16.32 milliequivalents/liter. The highest value was recorded for sample 13, while the lowest value was for sample 15.
The concentration in the lower layer ranged between 0.37 and 6.60 milliequivalents/liter. The lowest value was recorded for sample 15, while the highest value was for sample 21. The results of the chemical analysis showed a decrease in the concentration of potassium ions in the lower layer.
As for uncultivated soils, the concentration of potassium in the surface layer ranged between 1.00 and 75.95 milliequivalents/liter for samples 26 and 1 respectively. While the concentration of potassium ions in the lower layer ranged between 1.88 and 13.60 milliequivalents/liter for samples 14 and 6 respectively.
The study also showed a high concentration of potassium ions in the crust, as the values ranged between 1.92, 119.70 milliequivalents/liter, and the lowest value was recorded for sample 17, while the highest value was recorded for sample 23. According to Al-Domi et al. (1996), the concentration of potassium in the crust is higher than the usual range in the saturated soil extract.
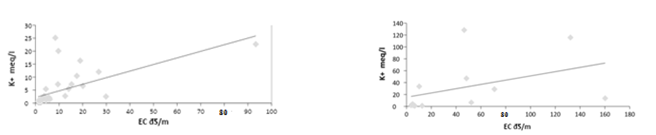
From the results of the statistical analysis, it was found that the concentration of potassium cation in cultivated and uncultivated soils has highly significant differences (p<0.05), and there are also highly significant differences between the results obtained for the same sites for potassium cation (p>0.05) for the depths studied. There is also a weak correlation between the potassium ion concentration and the electrical conductivity values of the saturated soil paste extract for cultivated and uncultivated soil samples, as shown in Figure 6 and Figure 7 with a value of 0.385 and 0.175, respectively.
3) Calcium
The results of the chemical analysis of the samples showed that the value of calcium in the surface layer of the cultivated soil ranged between 1.90 and 60.48 milliequivalents/liter. The lowest value was recorded for sample 17, while the highest value was recorded for sample 21. As for its concentration in the soil of the lower layer, it ranged between 2.00 and 43.15 milliequivalents/liter for samples 16 and 21, respectively. As for the uncultivated soil, its concentration in the surface layer ranged between 3.89 and 234.24 milliequivalents/liter. The lowest value was recorded for sample 26, while the highest value was for sample 20. In the soil of the lower layer, it ranged between 9.00 133.38 milliequivalents/liter for samples 14 and 20, respectively.
While the concentration in the crust layer extracts ranged between 9.60 and 490.01 milliequivalents/liter for samples 17 and 23 respectively, through chemical analyses, it is clear that calcium ions are present in uncultivated soils at high concentrations due to the lack of irrigation operations, as well as due to the dry and hot climate conditions throughout the year and the limited rainfall rates that are not sufficient to dissolve the lime and wash it from the soil profile. These results are consistent with what was indicated by Al-Mahi (1996b).
The results of the chemical analysis of soil samples showed the presence of calcium ions at high concentrations in the study area, which is consistent with what was indicated by Abu Dahi and Al-Younis (1988), who stated that soils in dry and semi-dry areas contain large amounts of calcium ions formed from limestone and sandstone, which are characterized by hardness. It is worth noting that the presence of calcium in the soil helps to stabilize the structure and cohesion of the soil.
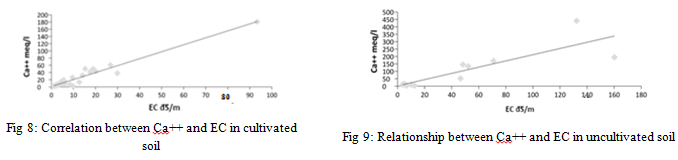
The results of the statistical analysis show that there are highly significant differences in the concentration of calcium cation in cultivated and uncultivated soil (p<0.05), and there are also highly significant differences between the results obtained for the same sites for calcium cation (p>0.05) for the depths studied. There is a strong correlation between the concentration of calcium ions and the electrical conductivity values of the saturated soil paste extract for cultivated and uncultivated soil samples as shown in Figure 8 and Figure 9 by 0.948 and 0.718 respectively.
H. Negative Ions
1) Chlorine
The results show that the concentration of chloride ions in uncultivated soils is higher than in cultivated soils. Its increase in the surface layer is due to the upward movement of water and evaporation-transpiration processes, as well as the dominance of chloride ions in most of the soils studied, as the ratio of sulfates to chlorides is <1, except for samples 21 and 22 respectively, which indicates that the soils under study have reached the final stages of salinization (chlorides) [31].
The high concentration of chloride ions in the soil can lead to a toxic effect on plants. The effect of chloride ions on plants is through physiological changes and low energy production, in addition to high accumulations of sodium chloride, which reduces the permeability of nutrients such as potassium and magnesium [32, 33].
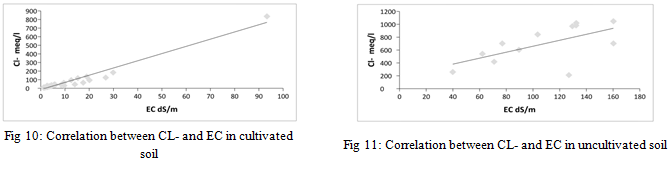
From the results of the statistical analysis, it is clear that there are highly significant differences in the concentration of chloride ions in cultivated and uncultivated soils (p<0.05), and there are also highly significant differences between the results obtained for the same sites for chloride ions (p>0.05) for the depths studied. There is also a strong correlation between the concentration of chloride ions and the electrical conductivity values of the saturated soil paste extract for cultivated soil samples, and there is a weak correlation for uncultivated soils, as shown in Figure 10 and Figure 11, with an amount of 0.954 and 0.384 respectively.
2) Sulfates
The results of the chemical analysis of soil samples showed high concentration of sulfate in most of the soils under study, as its concentration in the surface layer of cultivated soils ranged between 3.90, 289.16 mmEq/L, the lowest value was for sample 17 while the highest value was for sample 7. As for its concentration in the soil of the lower layer, it ranged between 1.50, 106.00 mmEq/L for samples 9, 21 respectively.
As for the surface layer of uncultivated soils, the values of sulfate ranged between 9.51 and 618.10 milliequivalents/liter, the lowest value was for sample 26 while the highest value was for sample 1. While the values for the lower layer ranged between 4.12 and 100.26 milliequivalents/liter for samples 24 and 20, respectively.
The results also showed a high concentration of sulfate in most of the soils studied in the surface layer and crust. Its concentration in the crust ranged between 8.55 and 553.90 mmEq/L for samples 14 and 23, respectively. This indicates a high concentration of sulfate in most uncultivated soils compared to cultivated soils, as sulfate accumulation in the soil increases under conditions of low rainfall rates and limited washing.
Soils in dry areas may acquire dissolved sulfate and other salts from water and the parent material [29], which is completely consistent with the study area in terms of dry climate.
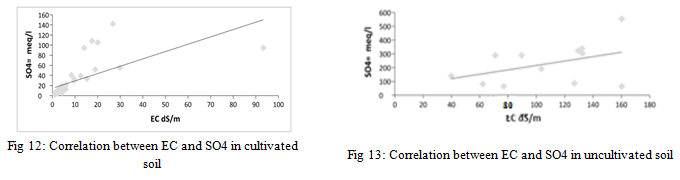
From the results of the statistical analysis, it is clear that there are highly significant differences in the concentration of sulfate ions in cultivated and uncultivated soils (p<0.05), and there are also highly significant differences between the results obtained for the same sites for sulfate ions (p>0.05) for the depths studied. There is also a correlation between the concentration of sulfate ions and the electrical conductivity values of the saturated soil paste extract for cultivated and uncultivated soil samples, as shown in Figure 12 and Figure 13, with an amount of 0.428 and 0.175 respectively.
3) Carbonates
The results of the chemical analysis of the soils showed a low concentration of carbonates, as the results showed the absence of carbonate concentrations in most of the soils under study except for sample 24, in which the carbonate concentration in the lower layer was 0.02 milliequivalents/liter. These results are consistent with what was indicated by Al-Mahi (1996), who noted the low presence of carbonates in the soils of arid and semi-arid regions.
4) Bicarbonate
The results show that the concentration of bicarbonate in the soils studied is low, but it falls within the normal range for soils, which is consistent with what was indicated by Al-Domi et al. (1996).
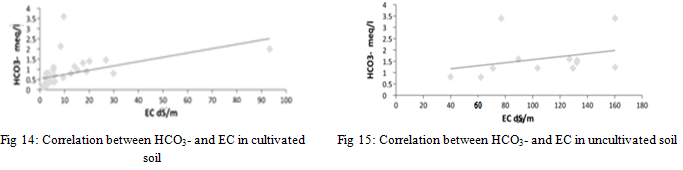
From the results of the statistical analysis, it became clear that the concentration of bicarbonate ions in cultivated and uncultivated soils does not show any significant differences (p<0.05). It was also clear that there are highly significant differences between the results obtained for the same sites for bicarbonate ions (p>0.05) for the depths studied. On the other hand, there is no correlation between the concentration of bicarbonate ions and the electrical conductivity values of the saturated soil paste extract for cultivated and uncultivated soil samples, as shown in Figure 14 and Figure 15 by an amount of 0.230 and 0.090 respectively.
The results of the study also showed that the ratio between (carbonates + bicarbonates) / (sulphates + chlorides) < 1 in some soils of the study area. This is consistent with Jawad and Tawfiq (2002), who indicated that when this ratio is reached, the soil is in a stage of intense adsorption of sodium by exchange complexes and displacement of calcium and magnesium. As a result, carbonate and bicarbonate ions are precipitated in the form of calcium or magnesium carbonate. The medium is also characterized by the dominance of sulphates and chlorides [31].
I. Exchangeable Sodium Percentage (ESP %)
The results of the chemical analysis of soil samples showed that the percentage of exchangeable sodium (ESP) for the surface layer of cultivated soils ranged between 2.12 and 30.74%, where the highest value was recorded for sample 25, while the lowest value was recorded for sample 22. In the lower layer, the values ranged between 1.33 and 19.17% for samples 22 and 19, respectively.
As for the uncultivated soils, the values for the surface layer ranged between 39.28 and 10.46%, the lowest value was for sample 26, while the highest value was recorded for sample 20. In the lower layer, the values ranged between 4.65 and 32.25% for samples 23 and 20, respectively.
The results of the chemical analysis show that the ESP values were higher than 15% for most of the soils studied, and these results are consistent with what Osman et.al. Found. (2003) who mentioned the presence of changes during the soil profile in the ESP values for the different depths of the soils he studied. These results show deterioration in the surface layer of the soils under study that was noticeable for the uncultivated soils.
And the results also show an increase in the ESP values for the surface layer of the soil compared to the lower layer due to the high concentration of exchangeable sodium relative to the rest of the other exchangeable ions.From the results of the statistical analysis, a table showed that there were no significant differences in the ESP values between cultivated and uncultivated soils at the significance level (p<0.05), but it was shown that there were highly significant differences between the results obtained for the same sites for the ESP values (p>0.05) for the depths studied.
Ben Mahmoud (1995) indicated that exchangeable sodium in sandy soils has no clear effect on most agricultural crops due to the low values of the cation exchange capacity of the soils, which means that the amount of exchangeable sodium in these soils is insufficient, and perhaps this explains the continuation of agriculture in these soils.
However, the study shows that continuing to follow unsound agricultural systems that are not compatible with the nature of the soil in the region and its climate may lead to soil deterioration due to the accumulation of sodium in it. The results of the study also show a high sodium content in uncultivated soils compared to cultivated soils, which indicates the presence of undesirable chemical and physical conditions in the soil. Exchangeable sodium works to break down soil particles in a way that does not allow the formation of stable aggregates and often disperses the soil due to the high hydration nature of sodium ions and the thickness of the water layer, as it does not compact strongly on the surfaces of clay, which causes a state of chemical imbalance in the soil, which reduces the chances of plants absorbing potassium, calcium and magnesium [41]. However, the high calcium and magnesium content of the soil reduces the permeability problems of the soils of the study area.
J. Organic Matter OM %
The results of the chemical analysis of the soil samples showed a decrease in the organic matter content of the soil, as the values ranged between 0.06% and 1.18%. The lowest value was recorded for sample 11, while the highest value was for sample 26. These values are consistent with what was indicated [9], who stated that the organic matter content of dry soils does not exceed 1%. The reason for the decrease in the percentage of organic matter is due to the harmful effect of salts in the soil on plant growth in terms of quantity and quality, as humus levels in soils affected by salts decrease to low levels, which is consistent with what was stated [35].
The results of chemical analyses also show that the organic matter content of cultivated soils is relatively higher than that of uncultivated soils, and the percentage of organic matter decreases with increasing depth, which is consistent with what was indicated [37 36]. This is due to the role played by plants in the formation of organic matter to varying degrees depending on the type of plant [38].
In the uncultivated soils, the percentage of organic matter in the surface layer ranged between 0.195% and 1.18% for samples 6 and 26 respectively, and 0.100% and 0.24% for samples 24 and 28 respectively for the lower layer. In general, it is noted that the percentage of organic matter in the uncultivated soils decreases with depth, as the texture of the soil, vegetation cover and climate play an important role in determining the percentage of organic matter, which is significantly affected by the soil salt content [40, 39].
As for the crust layer, the results of the chemical analysis show a difference in its content of organic matter, as the recorded values ranged between 0.21 and 3.38%. The lowest value was for sample 13, while the highest value was recorded for sample 30. It was found to be higher in areas that contained organic waste. Animal and plant life accumulated within the salt layer.
K. Calcium carbonate
The chemical analysis results of the samples showed that the value of calcium carbonate ranged between 0.30 to 12.90%, where the lowest value was recorded for sample 1 while the highest value was for sample 22, and the results indicate that the soil content of calcium carbonate is close.
The results of the chemical analysis of the soil also show that the crust content of calcium carbonate in the study area was high compared to the layers that follow it, where the values ranged from 0.89 to 29.00% for samples 1 and 26 respectively, and these values are close to what was indicated by Bin Mahmoud (1995) where he found that the high percentages of calcium carbonate in one of the soil horizons form a calcareous horizon characterized by low permeability, especially when the percentages reach 25%, which may explain the presence of a solid layer.
L. Cation Exchange Capacity (CEC)
The results of the chemical analysis of soil samples showed that the value of the exchange capacity of positive ions ranged between 1.24 and 10.00 milliequivalents/100 grams, where the highest value was recorded for sample 21 at a depth of 30-60 cm, while the lowest value was recorded for sample 30 at the same depth.
The results also showed a decrease in the exchange capacity of positive ions for most soils in the study area, as it did not exceed 5 milliequivalents/100 grams, except for samples 18, 19 and 22, whose values were 7.26, 6.01 and 6.10 milliequivalents/100 grams, respectively, because most of the soils were characterized by a coarse texture and a high sand content, which is consistent with what was indicated by Ben Mahmoud (1995), who stated that the exchange capacity of sandy soils does not exceed 5 milliequivalents/100 grams.
While the value of the exchange capacity of positive ions in the surface layer of the cultivated soil ranged between 2.39, 9.60 milliequivalents/100 grams for samples 13, 21 respectively, while in the lower soil samples it ranged between 2.00, 10.00 milliequivalents/100 grams for samples 16, 21 respectively.
The results also show a high value of the exchange capacity of positive ions for sample 19 due to the texture of the sandy loam soil and its containing higher proportions of clay minerals that work to increase the exchange surfaces of the soil, which is consistent with what was indicated by Iesch (2005) Corwin and who stated that the value of the exchange capacity of positive ions depends directly on the texture of the soil and its content of clay minerals and organic matter.
While the results of the chemical analysis of soil samples showed a decrease in the exchange capacity of positive ions in the uncultivated soils, where the values for the surface layer ranged between 2.81 and 4.70 milliequivalents/100 grams for samples 30 and 26, respectively, while the values for the exchange capacity of positive ions in the lower layer were between 1.24 and 3.92 milliequivalents/100 grams for samples 30 and 26, respectively. Soils with a low content of organic matter have a lower ion exchange capacity compared to the same soils that contain a higher percentage of organic matter, and organic colloids have a positive ion exchange capacity of about (100-200 milliequivalents/100 grams), which is consistent with what was indicated by Al-Shawk and Abd, (1990).
Conclusion
Most of the soils under study in the area have a sandy or sandy-silty or sandy texture. In general, the soils in the study area are characterized by a light color due to the presence of salts on the surface layer, especially in low-lying areas. Some soils are also characterized by the presence of impermeable layers at depths of 25-60 cm and sometimes at a greater depth. The organic matter in the soils of the study area was low, as it did not exceed 1.18%, and in contrast, the values of the cation exchange capacity of positive ions did not exceed 10 milliequivalents / 100 grams of soil for most soils. The results of the chemical analysis showed a higher electrical conductivity of the soils of the surface layer compared to the subsurface layer, as the values generally ranged between 1.03 - 107.91 dS.m-1, while its value in the crust layer was higher, as it ranged between 5.86 - 160.25 dS.m-1. The study showed that the region is prepared for the spread of the salinity phenomenon because it is located in a dry climate characterized by high temperatures and low rainfall, which causes the problem to worsen. It is also clear that the ratio CO3 + HCO3 / CL + SO4 was <1, which is evidence that the soil is in a stage of intense adsorption of sodium, and the ratio of sulfates / chlorides was <1 for all soils except samples 21, 22 and 29, which indicates the dominance of chloride salts, which means that the soils have reached the final stages of salinization, as the results of the statistical analysis reinforced these results. The results showed that ESP values were higher than 15% for most soils, as the values ranged between 1.33 and 30.74, and its value in the surface layer was higher than the lower layer of the soil. It was also found that 73.33% of the total soil samples under study were affected by salinity, as 63.33% of them were saline-sodic, including all uncultivated soils and about 55.55% of cultivated soils.
References
[1] GOOSSENS, Rudi; VAN RANST, Eric. “The use of remote sensing to map gypsiferous soils in the Ismailia Province (Egypt)”. Geoderma, 1998, 87.1-2: 47-56.? [2] Sosa, M. A, Martinez, H.P. and Avendano, M.J. Tropical grass production in Southeastren Bulagaria in realation to Culture Fertilizer application and data of Seedling, .J.P.L. 1982, Sci. 46:195-203. [3] Hydrological study of the groundwater reservoir of the Mizdah Formation (Upper Cretaceous) in the Sukna area by the General Water Authority, Janzour, 2000. [4] PAGE, A. L., et al. “Methods of soil analysis. Part 2. Chemical and microbial properties”. American Society of Agronomy, Madison, Wiscosin, USA, 1982.? [5] Claridge, G.G.C, and Campbell, I.B. “A comparison between hot and cold desert soils and soil processes”, In Aridic soils and Geomorphic processes, D.H. Yaalon (ED). Catena supplements I. Cremlingn, FRG, 1982, PP. 1-29. [6] Atinson, K. and Wangh, B. Morphology and Mineralogy of arid desert soil in Libya Sahara, Eaeth Surface process, 1979, 4: 103-115. [7] SWINDALE, L. D. “Distribution and use of arable soils in the semi-arid tropics”. In: Managing soil resources. Plenary Session Paper, Trans. 12th International Congress of Soil Science, New Delhi. 1982. p. 67-100.? [8] Vial, C.D, and Wright, C.Soil of Arid Zones of Chile, FAO Soils Bulletin No. 1, Rome, 1965, P. 42. [9] Bin Mahmoud, et al. Study of soil in the field, Al-Fateh University Publications, 1984. [10] Technical reports of wells by the German company (Philipp Holzmann, 1974) for the Al-Farjan Agricultural Project in the Sukna area. [11] Al-Battikhi, Anwar and Khatari Sayed. Soil Science: Principles and Applications, (Translated Book Authored by: Hausenweiler) Publications of Dar Al-Basheer - Al-Risala Foundation, 1999. [12] Al-Domi, Muhammad Fawzi. Soil Science: Fundamentals and Applications. (Translated book, authored by: Hausenweiler), Dar Al-Basheer Publications. Al-Risala Foundation, 2000. [13] Al-Darbi, Ali bin Muhammad Turki, Fundamentals of Soil Physics, (translated book authored by: Hillel Daniel), King Saud University, 1995. [14] Al-Aqidi, et al. Soil Morphology, Ministry of Education and Scientific Research, University of Baghdad, Beit Al-Hikma, 1989. [15] IMBUFE, et al. Effects of potassium humate on aggregate stability of two soils from Victoria, Australia. Geoderma, 2005, 125.3-4: 321-330.? [16] Al-Domi, et al. Methods of Analysis of Soils, Plants and Water. (Translated book by: Homer, D. Shaiman and Parker, F. Pratt) Publications of Omar Al-Mukhtar University. Al-Bayda- Libya, 1996. [17] Guidi, G. Relationships between organic matter and physic-chemical properties of soil, Vienna, 1980, October 21-23. D Publisher. [18] Ismail, Laith Khalil. Irrigation and Drainage, University of Mosul, Iraq, 1988. [19] Brady, N.C. The nature and properties of soil, 8th Ed, MacMillan Publishing Co. Inc. New York. (1982). Saline and Sadic Soils, Springler Verlag. Berline, 1974. [20] Nikos J. et al. The Basics of Salinity and Sodicity Effects on Soil Physical Properties, Geoderma, 2003. [21] Jawad, et al. Types of salts and degree of salinity of some Wadi Al-Shati soils, First National Conference on Building Materials and Structural Engineering, Scientific Research Journal, Faculty of Engineering and Technology - Sabha University, 2002. [22] CEMEK, Bilal, et al. “Assessment of spatial variability in some soil properties as related to soil salinity and alkalinity in Bafra plain in northern Turkey”. Environmental monitoring and assessment, 2007, 124: 223-234.? [23] ARDAHANLIOGLU, Osman, et al. Spatial variability of exchangeable sodium, electrical conductivity, soil pH and boron content in salt-and sodium-affected areas of the Igdir plain (Turkey). Journal of Arid Environments, 2003, 54.3: 495-503.? [24] BROWN, P. L., et al. Saline-seep diagnosis, control and reclamation. 1983.? [25] Al-Jawhari, Yousry. North Africa: A Study in Historical and Regional Geography, Alexandria, University Youth Foundation, 1970. [26] Abu Luqma, et al. The Jamahiriya: A Study in Geography, First Edition, Jamahiriya House for Publishing, Distribution and Advertising, Jamahiriya, 1995. [27] Ashraf, M. Breeding for Salinity Tolerance Proting in plant. Crit. Rev. Plant Sci. 1999, 13:17-42. [28] Lolyd, Seatz and Peterson. Acid Alkaline. Soil and Sodic Soil, From Chemistry of the soil, ed. F. Bear, 1964. [29] Al-Mahi, et al. Semi-arid and desert lands, (A translated book written by: Ascogni. J). Volume One, First Edition, Publications of Omar Al-Mukhtar University, Al-Bayda, 1996a. [30] Abu Dhahi, et al. Plant Nutrition Guide. University of Baghdad - Iraq, 1988. [31] Al-Zubaidi, Ahmed Haider. Soil Salinity, Theoretical and Applied Foundations, University of Baghdad, 1989. [32] Awad, Kazem Mashhout. Principles of Soil Chemistry, Ministry of Higher Education and Scientific Research, University of Mosul, Iraq, 1989. [33] Houat, D.R Acceptable Salinity Sodicity and pH Values, for Boreal Forest Reclamation, Alberta Environment, Environment Sciences Division Edmonton Alberta. 2000, Report ESD/LM/00-2. ISBN 0-7785-11, 37 – 1 (printed edition). [34] Ben Mahmoud, Khaled Ramadan. Libyan soils (their composition, classification, properties, and agricultural potential), National Authority for Scientific Research, 1995. [35] KAUSHIK, A., et al. Impact of long and short term irrigation of a sodic soil with distillery effluent in combination with bioamendments. Bioresource technology, 2005, 96.17: 1860-1866.? [36] Kenan, K. and Sinan, K. Spatial Variability and Alkalinity of a field having Calination risk in Semi-arid climate in Northern Turkey, Department of Soil Science, Faculty of Agriculture, University of Gaziosmapa, Sa, Ta, Sli, Cif+lik 60250 Tokat Turkey, 2006. [37] Sakla, Charles Shukry. Irrigation and Drainage Engineering, Faculty of Engineering - Mansoura University, 1991. [38] Stephen, John Ryan and Abdul Rashid. Soil and Plant Analysis Laboratory Manual, International Center for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria and National Agricultural Research Center (NARC), Islamabad, Pakistan, 2003. [39] LI, Xiaogang, et al. Decomposition of maize straw in saline soil. Biology and Fertility of Soils, 2006, 42: 366-370.? [40] SLAVICH, P. G.; PETTERSON, G. H.; GRIFFIN, D. Effects of irrigation water salinity and sodicity on infiltration and lucerne growth over a shallow watertable. Australian Journal of Experimental Agriculture, 2002, 42.3: 281-290.? [41] CORWIN, D. L.; LESCH, S. M. Characterizing soil spatial variability with apparent soil electrical conductivity: I. Survey protocols. Computers and electronics in agriculture, 2005, 46.1-3: 103-133.? [42] Al-Shawk, Arkan Mahmoud and Abdul, Mahdi Abdul-Kazem. The relationship between soil, water and plants. Ministry of Higher Education and Scientific Research. Technical Institutes Authority - Iraq, 1990. [43] Guthrie, R.L. Distribution of great groups of aridisols in the United States, In Aridic Soils and Geomorpic Processes, D.H. Yaalon (Ed.), Catena Supplement 1. Cremlingen, FRG, 1982, PP.29- 36. [44] El-Swaify, S.A, Walker, T.S, and Virmani, S.M. Dryland Management Alternatives and Research Need for Alfisols in the Semiarid Tropics, International Crops Research Institute for the Semiarid Tropics, Patancheru, P.O. Andhra Pradesh, India, 1984. [45] Marc, P. and Jacques, G. Handbook of Soil Analysis Mineralogical, Organic and Inorganic Methods, Updated English version, Berlin Heidelberg New York, 2006. [46] Al-Shaqweer, Muhammad Hammad Attia and Abdel-Hafiz, Abdul-Nasser Amin Ahmed. Theoretical Land Reform - Fayoum University, 2009. [47] Dograma Ji, Jamal Sharif. Introduction to Soil Physics, (Translated book by: Hillel Daniel), Ministry of Higher Education, University of Baghdad, 1990.
Copyright
Copyright © 2024 Hanan Mohamed Nasr, Abdulsalam Salem Mohamed. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64955
Publish Date : 2024-11-03
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

