Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Solar Photovoltaic Based Water Electrolysis System to Generate Hydrogen and Oxygen
Authors: Roger Coutinho, Zoltan Misquitta, Alan Rodrigues, Alywn Peter Arogyaswamy, Sandeep Sabnis
DOI Link: https://doi.org/10.22214/ijraset.2022.47242
Certificate: View Certificate
Abstract
In this never-ending quest for finding an alternative or a solution to the current problems related to increasing energy demands and climate change, various research, progress and development have been made in the subject of environmentally friendly energy systems. On one aspect the conversion of water into hydrogen gas and oxygen gas by using electro-chemical process is an ideal method which can produce a clean and green fuel via: Sustainable methods and fossil free path ways. Hydrogen production by means of using various renewable energy sources such as solar energy, wind energy, tidal energy and hydro energy is a way to eliminate dependency of three systems on the electric grid. In this study which is based on a technique in which a fuel cell (ox-hydrogen electrolyzer) is connected to a Photovoltaic solar panel to produce clean hydrogen and oxygen gas as a fuel. The objective of this project was to develop a strategy and make this generation process independent of other energy sources. A DC converter is used in such a sequence which can control and supply maximum power to the electrolytic cell which is connected to the solar Photovoltaic panel, which in turn we can obtain maximum hydrogen gas. Also, various catalysts which are most commonly found or easily available in the market are experimented in this research to get optimum output. The converter and voltage regulator is also responsible for operating the electrolytic cell in its optimum functioning region to avoid over heating of the cell. The DC converter and the Electrolytic cell has been tested and simulated within indoor and outdoor conditions. Analysis of this setup based on maximum power tracking showed 85% efficiency.
Introduction
I. INTRODUCTION
The conventional fuels which we are using today will eventually run out, so we need alternative sources of energy for our needs. Conventional fuels create a lot of pollution but hydrogen gas is a clean fuel [1] [3]. It does not produce any harmful gases or by products after burning. Hydrogen gas produced in large quantities can be compressed safely after purification. This compressed gas can be stored in the tanks occupying lesser area for further use as a fuel or to generate back electricity. As for now hydrogen production is mostly done from fossil fuel which results in carbon emissions [2]. So, hydrogen via solar energy has no harmful emissions or byproducts Oxygen will be obtained in the process and can be used for medicinal uses after filtering and purification. Thus, our project aims to create clean hydrogen and oxygen from electrolysis of water using the electricity generated from Photovoltaic solar panels. However, hydrogen generation by electrolysis of water has a share of only 4% in global production as electricity cost is the largest fraction of the production cost [2]
Electrical current runs through the electrolyte when a specific voltage is given to the electrodes, and water splits into hydrogen and oxygen at the cathode and anode electrodes, respectively. The lowest voltage required for water breakdown is 1.23 V, but in practice, more voltage is required to overcome system inefficiencies; this additional voltage is referred to as over potential. Electrical current runs through the electrolyte when a specific voltage is given to the electrodes, and water splits into hydrogen and oxygen at the cathode and anode electrodes, respectively. The lowest voltage required for water breakdown is 1.23 V, but in practice, more voltage is required to overcome system inefficiencies; this additional voltage is referred to as over potential [3] [4].
A redox reaction occurs mainly in pure water at the cathode which is negatively charged, with electrons being delivered to hydrogen cations to generate hydrogen gas. Similarly, on the other part an oxidation reaction happens at the anode which is positively charged, generates oxygen gas and supplying electrons to the anode [5].
The equation is as follows:
“Cathode (reduction): 2 H2O (l) + 2e− → H2 (g) + 2 OH− (aq)
Anode (oxidation): 2 OH− (aq) → 1/2 O2 (g) + H2O (l) + 2 e−”
II. MATERIALS AND METHODS
The objective here is to:
- Fabricate a solar PV based water electrolysis system to generate hydrogen and oxygen
- Research and development in its storage
- Research as using hydrogen as a fuel cell
- Experiment and analyze injection of hydrogen or blending of hydrogen in various fossil fuels used in IC engines
- Research and analyze domestic use of hydrogen
A. Material Used
For fabrication of the hydrogen fuel cell we felt that these were the ideal materials which could be used. Also, by keeping a point in mind that it doesn’t react with the solution or fail under working condition or various external factors affecting the cell.
- Acrylic sheet – used for end plate
- EPDM Rubber – for the gasket or rubber rings
- 316 Stainless Steel – for Anode & Cathode
- 3D printed disk (polycarbonate) – separator
- Polyurethane pipes – supply of solution and gas from the fuel cell
- Elbows, pipe joints, reducers, valves, etc.
B. Stack Assembly
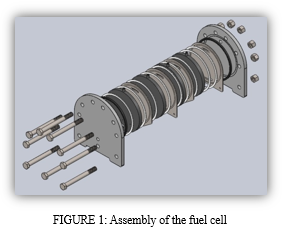
- Screw 9 bolts into the end of the acrylic plate and secure it to a bench.
- Then place the rubber ring or the silicon ring to the inner side of the plate
- Then place the SS 316 metal plate on top
- After that place another rubber ring on top of that
- Place the 3D printed part on top of the metal plate
- (This is the completion of one cell)
- Then place a rubber ring
- Similarly, on top of the rubber ring place the metal plate
- Again, place the rubber ring on top of the metal plate and then place the 3D printed part. (This completes 2 cells which make one stack)
Fuel cell stacks with total 4 cells were built to generate and separate hydrogen and oxygen by using electricity. The system uses a DC power supply. The negative terminal of the system is connected to the cathode of the fuel cell.
Similarly, the positive side of the power supply is connected to the anode or the positive terminal of the fuel cell [7].
C. Fuel Cell Working
The solution is added to the 2 containers which are provided, each for oxygen gas and Hydrogen gas. This solution is made to flow through the pipes into the fuel cell.
The stacking of the cell is made in such a manner as per the requirement or the quantity of the gas required.
One anode and one cathode plate are linked to the positive and negative terminals of the power supply in a single stack.
On the positive side (anode) we will observe oxygen gas forming, similarly on the negative side we can observe hydrogen gas forming. The quantity of hydrogen gas which is generated per unit time is directly proportional to the current flowing through the electrolyte [6]. The gas which is generated on the side of anode and the cathode is separated by the solid 3D printed disk which does not allow the gas to mix. The accumulated gas is taken out from the nozzle on top of this 3D printed disk.
The gas can be further stored or passed for purification process and used later.
III. TEST AND ANALYSIS
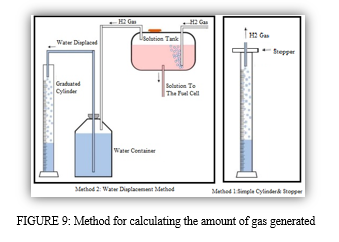
To understand the basic concept and to study the reactions by using various chemicals and their outputs along with their byproducts, we constructed a Hoffman’s voltammeter. It is commonly used as a small-scale electrolytic cell, in our experimental investigation and understanding. It is made up of three upright cylinders that are connected together. Water and electrolyte can be added through the top of the inner cylinder. Platinum, graphite, and other noble metals are commonly used. Instead, we used stainless steel rods 316 and 316L
At the bottom of each of the two side cylinders, the electrode is connected to the positive and negative terminals of an electrical source.
When electricity is conducted through the Hoffman volt ammeter, gaseous oxygen develops at the anode (positive) and gaseous hydrogen forms at the cathode (negative). Each gas bubble generated on the plate displaces water, which accumulates in the above two outer tubes, where a stopcock can be used to remove it.
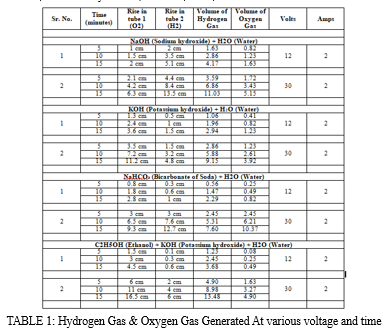
The various solutions tested:
- NaOH (Sodium hydroxide) + H2O (Water)
- KOH (Potassium hydroxide) + H2O (Water)
- NaHCO? (Bicarbonate of Soda) + H2O (Water)
- C2H5OH (Ethanol) + KOH (Potassium hydroxide) + H2O (Water)



V. FUTURE SCOPE & RESEARCH
When it comes to protecting our world, humanity has an uphill struggle. To avoid some of the worst impacts of climate change, we must keep global temperatures from climbing 1.5 degrees Celsius beyond pre-industrial levels. To achieve so, global carbon emissions must be zero by 2050. [8] . To attain this aim, a number of solutions will be required, but one technology that is gaining traction is green hydrogen. Green hydrogen is hydrogen that is created only from renewable energy sources. [1].
There are tremendous amount of studies and analysis on
- Storage of hydrogen gas:
a. Storage in large Tanks
b. Storage in cylinders
c. Storage underground (salt mines, depleted oil wells, underground caves) [9]
2. Use of hydrogen for power or electricity generation
3. Use as fuel of automobiles (combustion or for blends in gasoline and liquid fuels)
4. Used as fuel for gas or diesel power plants
VI. ACKNOWLEDGEMENTS
We express our gratitude to the department of mechanical engineering and the department of basic science and humanity for their endless support, guidance and help. We sincerely want to thank Prof. Sandeep Sabnis our project guide who has helped us greatly and guided us in gathering and providing the essential resources, which helped us to put the various ideas together and completing this project in the best possible way. We would like to thank our project coordinator Prof. Cleta Pereira, for the constant support and encouragement received. The mechanical workshop department in helping us fabricates the project, without their guidance and knowledge this wouldn’t be a success.
Conclusion
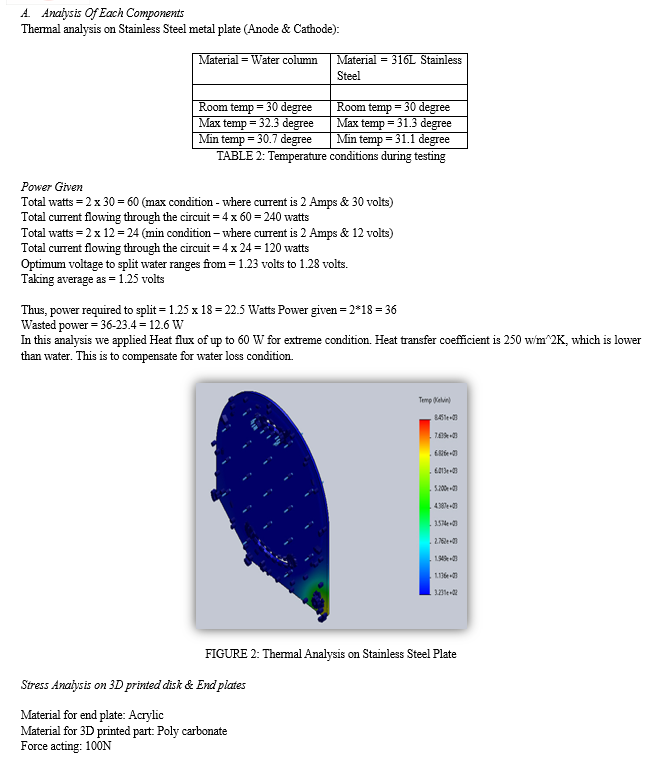
Based on the active surface area of the electrolytic cell, the appropriate current applied was found to be 30watts at max condition and 24 watts at minimum operating condition. 1) When a voltage of 12V is supplied across the stack, the gas production rate is calculated to be 0.95 LPM HHO gas approx. The hydrogen gas produced is 0.75 LPM, while the oxygen gas produced is 0.151 LPM. 2) As the result, the quantity of hydrogen gas produced is twice as much as the quantity of oxygen gas produced. This is due to the large number of mole transfers that occur during the electrolysis process [4] [6] 3) According to the thermal analysis performed using the solid works software no external cooling is required in the design because the maximum temperature rise in the electrode plate and water column is 31.3 C and 32.3 C, respectively. The amount of heat created in the cell is negligible. 4) The active surface area is a critical consideration when designing a cell since it dictates the maximum amount of current that can be applied without the cell overheating. The rate of gas production is then determined by the amount of current 5) The voltage is distributed via the neutral plates in between the electrodes. Anything above 2.2 volts is a waste of energy that leads to overheating and stainless-steel corrosion. As a result, neutral plates aid in efficient utilization. The overall total voltage flowing through the fuel cell is 60 watts and 4 cells makes it 240 watts
References
[1] “Fact Sheet, Fuel Cell & Hydrogen Energy Association” [2] Kaveh Mazloomi, Nasir b. Sulainam, Hossein Moayedi, “Electrical Efficiency of Electrolytic Hydrogen Production,” Int. J. Electrochemical. Sci., vol. 7 (2012), pp. 3314 – 3326, April 2012 [3] A. A. Lanjewar, et. al., “Design of HHO Cell Kit”, International Journal of Chemical Sciences and Research (IJCSR), vol (7), PP 1-8, 2017 [4] Emmanuel Zoulias, Elli Varkaraki, Nicolaos Lymberopoulos, Christodoulos N. Christodoulou, George N. Karagiorgis, “A Review on Water Electrolysis,” [5] “en.wikipedia.org/wiki/Electrolysis_of_water” [6] “Water Electrolysis & Renewable Energy Systems, Fuel Cell Today”, May 201 [7] Al Jumlat Ahmed, Md. Abdullah-Al-Munem*, Yasin Imam Arnab, “Developing Prototype and Performance Evaluation of a Hydrogen Generator”, January 2018. [8] “un.org/sustainabledevelopment/climate-change/” [9] U.Bünger, J.Michalski, F.Crotogino, O.Kruck, “Large-scale underground storage of hydrogen for the grid integration of renewable energy and other applications”, 2016 [10] J. Nowotny?, C.C. Sorrell, L.R. Sheppard, T.Bak. Solar-hydrogen: Environmentally safe fuel for the future. Centre for Materials Research in Energy Conversion, School of Materials Science and Engineering, University of New South Wales, Sydney, NSW 2052, Australia [11] “Rusdianasari Rusdianasari, Yohandri Bow, Tresna Dewi. HHO Gas Generation in Hydrogen Generator using Electrolysis” [12] “Z. Wang*, R.R. Roberts, G.F. Naterer, K.S. Gabriel. Comparison of thermochemical, electrolytic, photo electrolytic and photochemical solar-to- hydrogen production technologies. Clean Energy Research Laboratory, Faculty of Engineering and Applied Science, University of Ontario Institute of Technology, and 2000 Simcoe Street North, Oshawa, Ontario L1H 7K4, Canada” [13] “Pathways to electrochemical solar-hydrogen technologies. The Royal Society of Chemistry” [14] “Hydrogen Basics - Solar Production Florida Solar Energy Centre”
Copyright
Copyright © 2022 Roger Coutinho, Zoltan Misquitta, Alan Rodrigues, Alywn Peter Arogyaswamy, Sandeep Sabnis. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET47242
Publish Date : 2022-10-31
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

