Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
Study the Diversity of Nocturnal Moths in Agricultural Field of Western Maharashtra Region
Authors: Shekhar Phadtare, Vinod R. Ragade, Ravindra Gaikwad, Priyanka Takawane
DOI Link: https://doi.org/10.22214/ijraset.2024.58539
Certificate: View Certificate
Abstract
Study of moths is important as they are significant part of the ecosystem. A study on macro-moths was conducted at agricultural field of Western Maharashtra region from month of June 2022 to May 2023. The Main aim of study to acquire the detail information of moths found in agricultural filed of District Satara and Pune. During the study period, a total 2012 specimen’s moths were observed. In addition, the number of families recorded from agricultural field of Western Maharashtra is also high, 20 families and 57 species occurred in desired location. Agricultural field of Western Maharashtra shows moths diversity. So, moth diversity was studied from Western Maharashtra from two sites including Phaltan and Baramati taluka respectively. These two sites have a few species in common. In general, Arctiidae, Noctuidae, Pyralidae and Sphingidae dominated both the sites. The Moths were collected by using mercury light traps (160 W). The moth diversity has been studied for the first time from Agricultural field of Western Maharashtra region.
Introduction
I. INTRODUCTION
Biodiversity and natural resources form the root of all living system. India is fortunate enough to be ranked sixth among the twelve mega biodiversity country (Singh, 2004). Its biological resources include 50,000 species of plants and 81,000 species of animals including ones belonging to lower phylum. An insect, especially moths played an important role in earth ecosystems and has effect on the environment. Recent recorded report is over 1, 27, 000 species of moths found all over the world and over 12,000 species found in India (Alfred et. al. 1998). Human activity causes threaten to the moth diversity. Now a day’s moths are major agricultural pests in many parts of the world. Most of them found in grassland, agricultural and forest ecosystem. Light trapping of lepidopteron has been carried out widely in temperate and tropical regions throughout the world. The long- term status of insect faunas in Western Maharashtra region has attracted minimal research. Time frames and processes of change are poorly understood and have received scant investigation. Light-trapping measures are dependent on moth behavior and local flight variables (Southwood, 1978; Bowden, 1982) and such data have two numerical weaknesses: catches are not unit-area samples; and sampling bias is always present and seldom constant. Western region of Maharashtra State shows great animal diversity. It lies between 18o3’ N to 18o12’ N latitude and 74o13’ E to 74o40’ E longitude; 548 m above mean sea level.
II. MATERIALS AND METHODS
A. Site Study
Diversity study of moths was carried out in two Districts including Satara and Pune belongs to Western Maharashtra region, Maharashtra. It is located at 18.150 N 74.580 E. It is located in the rain shadow and therefore receives only around 400-500 mm of average rainfall in the monsoon. The water for irrigation is provided by ‘Veer Dam’ in both zones. Two regions selected for moth collection one was from Satara and another is from Pune District. The site one was Agricultural field of Phaltan taluka at south side of Satara and second site was Baramati taluka, both area are the agriculture ecosystem.
B. Specimen Collection and Observation by Light Trap Method
A mercury light trap method was used for the collection of moth. This is most common method of collecting nocturnal moths that hide or rest during the day in places where they are unlikely seen. Large number of moths caught at night using a light trap. White cloth screen (3 x 3.5) was hanging between two poles and extended forward over the ground slightly away from the direct source of mercury light placed.
Specimens are collected with the help of mercury light trap, (160 W) in two sites from District Satara and Pune in Western Maharashtra region. Some specimens were collected from a street lamp lights and on flowers during night by battery traps. Specimens were preserved in research laboratory.
C. Preservation of collected specimens
The dead moths were collected from the two different sites and kept in relaxation chamber. Relaxation process on moths were carried out in a relaxing jar. A relaxing jar, like a killing jar, should have a wide mouth and a tightly fitted lid. Place an absorbent layer in the bottom of the jar. Prepared the material with water and add a little ethyl acetate to inhibit fungus growth. Place a protective layer over the absorbent layer and place moths that need 24 hrs for relaxation. The pinning process was conduct after relaxation process. Pinning was started by inserting the half part of the pin into the center of thorax of moth. After pinning step, the moth specimen was placed into a spreading board, with wings of moth touch to the board. The small pins were used for spreading the wings at a 90° angle on the body. The forewing and hind wing spread on spreading board with the help of pins. The preservation and labeling process carried out after spreading process. The spread moth was preserved into oven at 37°C for 24 hrs. The specimen was labeled, which contain the location from where the specimen was obtained, the date when it was obtained, environment and the name of the collector. Use a permanent ink pen to write the labels. After 24 hrs incubation period, store the preserved moth specimens onto mounting board. After that, use the naphthalene balls for the storage process.
D. Identification of Specimens
The photographic collection as well as preserved specimens from both of sites was identified with the help of identification key, Google lens and available literature. The most of specimens were identified up to family levels.
III. RESULT AND DISCUSSION
In present study, average 167 specimens of moth per month were observed from two sites during June 2022 to May 2023. The total 869 specimens from site I located in Phaltan taluka, District Satara and a total 1143 specimens were observed from site II located in Baramati taluka, District Pune. The observation of specimens was conducted by using light trap method. The twenty families like Arctiidae, Bombycidae, Hyblaeidae, Nymhalidae, Noctuidae, Pyralidae, Sphingidae, Erebidae, Indarbellidae, Oecophoridae, Lymantriidae, Carposinidae, Eucleidae, Gelichidae,Pterophoridae, Psychidae, Eucosmidae, Pterophoridae, Cryptophae and Tortricidae were recorded (Table 1). The 50% families were recorded from both the sites. In fig. 2 shows the number of individuals belonging to each family from two sites in Western Maharashtra reagion. The highest numbers of moths were recorded in family Noctuidae, Pyralidae, Sphingidae and Arctiidae respectively.
Table No. 1 Total number of species were identified from different families of order Lepidoptera [Recorded (+) and Not Recorded (-)]
|
Sr. No. |
Family |
Site I |
Site II |
Total No. of Species |
|
1 |
Arctiidae |
+ |
+ |
05 |
|
2 |
Bombycidae |
+ |
+ |
01 |
|
3 |
Hyblaeidae |
- |
+ |
01 |
|
4 |
Nymhalidae |
+ |
+ |
01 |
|
5 |
Noctuidae |
+ |
+ |
19 |
|
6 |
Pyralidae |
+ |
+ |
12 |
|
7 |
Sphingidae |
+ |
+ |
05 |
|
8 |
Erebidae |
- |
+ |
01 |
|
9 |
Indarbellidae |
+ |
- |
01 |
|
10 |
Oecophoridae |
+ |
- |
01 |
|
11 |
Lymantriidae |
+ |
- |
01 |
|
12 |
Carposinidae |
+ |
+ |
01 |
|
13 |
Eucleidae |
+ |
+ |
01 |
|
14 |
Gelichidae |
- |
+ |
01 |
|
15 |
Pterophoridae |
- |
+ |
01 |
|
16 |
Psychidae |
+ |
+ |
01 |
|
17 |
Eucosmidae |
- |
+ |
01 |
|
18 |
Pterophoridae |
+ |
+ |
01 |
|
19 |
Cryptophae |
+ |
- |
01 |
|
20 |
Tortricidae |
+ |
- |
01 |
|
Total No. of Species |
15 |
15 |
57 |
|
The 19 different species were recorded from family Noctuidae. A total 57 species belonging to twenty families were identified from site I and Site II. The lowest number of specimens was recorded from some families including Bombycidae, Hyblaeidae, Nymhalidae, Erebidae, Indarbellidae, Oecophoridae, Lymantriidae, Carposinidae, Eucleidae, Gelichidae, Pterophoridae, Psychidae, Eucosmidae, Pterophoridae, Cryptophae and Tortricidae.
The species diversity were observed according to families (Table No.2). A total 15 families were recorded from site I (Phaltan Taluka) and 15 families from site II (Baramati Taluka). In both sites, the Noctuidae was the most abundant in both species richness and abundance. Pyrallidae was second most abundant family in agricultural field of both the sites. In this study, we also found a close relationship between plant species richness and moth diversity, the highest moth diversity recorded in vegetable field.
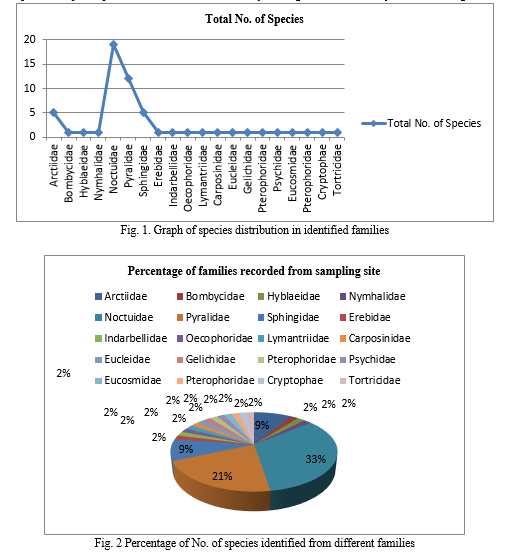
The moth morphological species richness was similar in both the habitats. The diversity of moths from both the site was 50-50 percentage. The overall structure of the vegetation community explains much moth diversity. Vegetation based communities are the major spatial divers of moth diversity in this landscape. The diversity of moth fauna in agricultural field of Western Maharashtra is mainly due to the rich, vegetation in this area as vegetation plays an important role for existence of lepidopteron fauna in a community as it provides the main source of food etc. for insects. Conservation of natural habitats is very essential for the existence of many species of lepidopteron. The total number of individuals caught in a light trap is an indication of biomass although more care has to be taken in its interpretation than for diversity as the size of a light trap catch can be influenced significantly by the setting of the trap, interference from other lights and lunar cycles (Barlow and Woiwod, 1989).
Table No. 2. Common name, scientific name, families and host plant of Identified specimens
|
Sr. No. |
Name of Species |
Common Name |
Family |
|
1 |
Rajendra perrottetii |
- |
Erebidae |
|
2 |
Syntomoidas imaon |
Handmaiden moth |
Arctiidae |
|
3 |
Syntomeida epilais |
Oleander moth |
Arctiidae |
|
4 |
Arctiinae |
Tiger moth |
Arctiidae |
|
5 |
Hippotion celerio |
Hawk moth |
|
|
6 |
Daphnis nerii |
Oleander hawk moth |
|
|
7 |
Acherontia atropos |
African deaths head hawkmoth |
|
|
8 |
Erebus macrops |
Owl moth |
Nymphalidae |
|
9 |
Asota caricae |
Tropical tiger moth |
Noctuidae |
|
10 |
Hyblaea Puera |
Teak defoliator |
Hyblaeidae |
|
11 |
Chrysodeixis includence |
Soybean looper |
Noctuidae |
|
12 |
Eudocima follonic |
Citrus fruit sucking moth |
Noctuidae |
|
13 |
Inderbelo quadrinotata |
Bark eating caterpillar |
Indarbellidae |
|
14 |
Tonia Ziyphi |
Citrus leaf roller |
Oecophoridae |
|
15 |
Chumetia transverso |
Shoot borer |
Noctuidae |
|
16 |
Bombtelia jacosatrix |
Leaf eating caterpillar |
Noctuidae |
|
17 |
Orthago exuvinaea |
Leaf webber |
Pyralidae |
|
18 |
Sylepta lunalis |
Leaf roller |
Pyralidae |
|
19 |
Euproctis fraternal |
Hairy caterpillar |
Lymantriidae |
|
20 |
Conogethes panctiferalis |
Fruit borers |
Noctuidae |
|
21 |
Achaeca jonata |
Fruit sucking moth |
Noctuidae |
|
22 |
Aganus ficus |
Leaf eating caterpillar |
Arctiidae |
|
23 |
Meridarchis scyrode |
Bee fruit borer |
Carposinidae |
|
24 |
Trymalitis margaritas |
Chickoo seed borer |
Tortricidae |
|
25 |
Nephopteryx eugraphella |
Chicko moth |
Pyralidae |
|
26 |
Opisina arenosella |
Black headed caterpillar |
Cryptophae |
|
27 |
Paras lepida |
Slug caterpillar |
Eucleidae |
|
28 |
Macalla moncausalis |
Leaf Webber |
Pyralidae |
|
29 |
Thylocoptila panrosema |
Nut borer |
Pyralidae |
|
30 |
Helicoverpa armigera |
Bud borer |
Noctuidae |
|
31 |
Deilephila nerri |
Army green moth |
Sphingidae |
|
32 |
Leucinodes orbonalis |
Brinjal shoot and fruit borer |
Pyralidae |
|
33 |
Phthorimaea operculella |
Potato tuber moth |
Gelichidae |
|
34 |
Agrotis ipsilon |
Potato cut worm |
Noctuidae |
|
35 |
Herse convolvuli L. |
Convolvulus hawk moth |
Sphingidae |
|
36 |
Euzophera perticella |
Brinjal stem borer |
Pyralidae |
|
37 |
Antoba olivacea |
Brinjal leaf roller |
Noctuidae |
|
38 |
Helicoverpa armigera |
Tomato fruit borer |
Noctuidae |
|
39 |
Euzophera perticella |
Tomato stem borer |
Pyrallidae |
|
40 |
Earias virtella |
Bhendi shoot and fruit borer |
Noctuidae |
|
41 |
Sylepta derogate |
Bhendi leaf roller |
Pyralidae |
|
42 |
Helicoverpa armgera |
Gram pod borer |
Noctuidae |
|
43 |
Etiella zinckenella |
Lentil pod borer |
Pyralidae |
|
44 |
Plutella xylostella |
Diamond black moth |
Plttelidae |
|
45 |
Hellula undalis |
Cabbage borer |
Pieridae |
|
46 |
Thysanopulsia orihalcea |
Cabbage semilooper |
Noctuidae |
|
47 |
Sphenarches caffer |
Bottle guard plume moth |
Pterophoridae |
|
48 |
Margaronia indica |
Snake gourd semilooper |
Pyralidae |
|
49 |
Cydia hemidoxa |
Top shoot borer |
Eucosmidae |
|
50 |
Dichocrocis punctiferalis |
Shoot, panicle and capsule borer |
Pyralidae |
|
51 |
Acanthopsyche bipar |
Bag worm |
Psychidae |
|
52 |
Spodoptera hexigua |
Army worm |
Noctuidae |
|
53 |
Spodoptera litura |
Cumin Caterpillar |
Noctuidae |
|
54 |
Amsacta morei |
Red hairy caterpillar |
Noctuidae |
|
55 |
Spilosoma obliqua |
Bihar hairy caterpillar |
Arctidae |
|
56 |
Agrotis ipsilon |
Greasy cutworm |
Noctuidae |
|
57 |
Helicoverpa armigera |
Fruit borer |
Noctuidae |
IV. ACKNOWLEDGEMENT
We would like to thank Principal Dr. Preeta Nilesh, KET’s V.G. Vaze ASC College, Mulund (E), Dr. B.B. Sharma KET’s Mumbai and Dr. S.S. Barve, Dy Director SRC, Dr. Ajit Kengar, HOD Botany Department KET’s V.G. Vaze College, Mulund East, Mumbai and Principal Dr. Bharat Shinde, Vidya Pratishthan’s Art’s, Science and Commerce College, Baramati for the encouragement and providing facilities.
References
[1] A.B. Sudeep, R.Khushiramani, S.S. Athawale, A.C. Mishra and D.T. Mourya (2005). Characterization of a newly established potato tuber moth (Phthorimaea operculella zeller) cell line. Indian J Med Res 121, pp 159-163. [2] B. Horvath (2013). Diversity comparison of nocturnal microlepidoptera communities (Lepidoptera: Macroheterocera) in different forest stands. Natura Somogyiensis. pp 229-238. [3] Cigdem Yilmaz and Hanife GenÇ (2012). Determination of the Life Cycle of the Olive Fruit Leaf Moth, Palpita unionalis (Lepidoptera: Pyralidae) in the Laboratory. Florida Entomologist. (95)1. [4] D.V. Stojanovic and Srecko B. Curcic (2011). The Diversity of Noctuid Moths (Lepidoptera: Noctuidae) in Serbia. Acta Zoologica Bulgarica. 63 (1): 47-60. [5] D. Adiroubance and P. Kuppammal (2010). Lepidopteran fauna of Agri-Horticultural ecosystem in Karaikal region. Journal of Biopesticides 3 (1 Special Issue) 001-010. [6] D. DavisA Review of the West Indian Moths of the Family Psychidae with Descriptions of New Taxa and Immature Stages. [7] E.G. White (1991). The Changing Abundance of Moths in a Tussock Grassland, 1962-1989, And 50- To 70- Year Trends. New Zealand Journal of Ecology, Vol. 15, No. 1. [8] G. Brehm (2005). Diversity and community structure of geometrid moths of disturbed habitat in a montane area in the Ecuadorian Andes. Journal of Research on the Lepidoptera. 38: 1-14. [9] G. Mathew et. al. (1993). Biodiversity in the Western Ghats - A Study with Reference to Moths (Lepidoptera: Heterocera) in the Silent Valley National Park, India). ENTOMON-1995, Vol. 20 (2): 25-33. [10] H. Noori, and J. Shirazi (2012). A Study on Some Biological Characteristics of Olive Leaf Moth, Palpita Unionalis Hubner (Lep: Pyralidae) in Iran. J. Agr. Sci. Tech. Vol. 14: 257-266 [11] H.S. Rose (2001). An Inventory of the Moth Fauna (Lepidoptera) of Jatinga, Assam, India. Zoos Print Journal 17(2): 707-721. [12] I. Kehimkar, Moths of India Book. [13] J. Kashefi, G.P. Markin and J.L. Littlefield (2008). Field studies of the biology of the moth Bradyrrhoa gilveolella (Treitschke) (Lepidoptera: Pyralidae ) as a potential biocontrol agent for Chondrilla juncea. XII International Symposium on Biological Control of Weeds. 568-572. [14] J. D. Palting (2013). Preliminary Assessment of the Moth (Lepidoptera: Heterocera) Fauna of Rincon de Guadalupe, Sierra de Bacadehuachi, Sonora, Mexico. USDA Forest Service Proceedings RMRS- P- 67:169- 172. [15] J. H. Itamies et. al. (2012). Climate Change and Shifts in the Distribution of Moth Species in Finland, with a Focus on the Province of Kainuu. Climate Change- Geophysical Foundations and Ecological Effects. pp 273- 296. [16] Kailash Chandra et al. (2013). Diversity of Hawk Moths (Lepidoptera: Sphingidae) in Veerangana Durgavati Wildlife Sanctuary, Damoh, Madhya Pradesh. Biological Forum – An International Journal 5(1): 73-77 [17] L.N. Kakati and B.C. Chutla (2009). Diversity and ecology of wild sericigenous insects in Nagaland, India. Tropical Ecology 50 (1):137-146. [18] Muhammad Aslam (2013). Checklist of moth fauna of Peshawar, Pakistan. Arthropods. Vol. 2 (4): 237-241. [19] M. Cook (2003). Changing views on melanic moths. Biological Journal of the Linnean Society. 69: 431–441. [20] P. Huemer(2009). Biodiversity of butterflies and moths in the National Park Hohe Tauern. 4th Symposium of the Hohe Tauern National Park Conference Volume for Research in Protected Areas. pp 135-136
Copyright
Copyright © 2024 Shekhar Phadtare, Vinod R. Ragade, Ravindra Gaikwad, Priyanka Takawane. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET58539
Publish Date : 2024-02-21
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

