Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
The Role of Gut Microbioma in Human Health: Exploring the Impact on Metabolism, Immunity, and Neurological Disorders
Authors: Ayushi Maurya, Sankarasubramanian Vidyameenakshi , Neerja Masih
DOI Link: https://doi.org/10.22214/ijraset.2024.64782
Certificate: View Certificate
Abstract
The human microbiota has been progressively accepted for its role in sustaining overall health and well-being. The gut microflora vigorously communicates with the host metabolic system that exert influence on the progression, maturation, immune cells development and immune homeostasis maintenance. For the proper functioning of the immune system a diversified and steady gut microflora is needed, while “Dysbiosis” or an imbalance in the gut microbioma will lead to autoimmune and inflammatory diseases. The gut microbiota provides shape to our immune response and contribute to host protection against pathogen or bacterium through mechanisms such as the transformation of pro- inflammatory cytokine production, regulatory T-cell inductor and by intestinal mucosaql barrier. In addition to gut microbiota can affect our neurological function and play a key role in the pathogenesis of psychological disorders such as autism, depression, anxiety, Parkinson and Alzheimer’s disease. Gut microbiota can regulate neurological disorders by supplementing them with fecal transplantation, probiotics and antibiotics. An important parameter to consider gut microbioma disorder is Firmicutes/ Bacteroidetes.
Introduction
I. INTRODUCTION
Since its discovery, various studies have underlined the importance of microbiota in health and disorders. Microbiota can be divided into four categories: gut, oral, respiratory, and skin microbiota, depending on the specific areas.
The microbial populations coexist harmoniously with the host, playing a crucial role in maintaining homeostasis and modulating immune response.
On the other hand, dysbiosis of the microbiota can result in diseases such as cancer, respiratory disorders, cardiovascular diseases (CVDs), and deregulation of body systems.[1]
The term "microbiome" refers to the collection of genomes from all the microorganisms in the environment, which comprises the microbial population as well as metabolites, structural components, and environmental factors.
The microorganisms in the stomach perform a number of tasks, including vitamin synthesis, pathogen defense, food fermentation, and immune response stimulation.
The six phyla that make up the gut microbiota are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with Firmicutes and Bacteroidetes being the two main groups. Candida, Saccharomyces, Malassezia, and Cladosporium are the most researched fungus (gut mycobiota).[2]
Most chronic diseases that affect people are closely associated with gut microbiota dysbiosis. Disturbances in the gut microbiota have a high correlation with diseases such as hepatocellular carcinoma, cirrhosis, obesity, metabolic syndrome, atherosclerosis, fatty liver disease (ALD), inflammatory bowel disease (IBD), hepatocellular carcinoma, and alcoholic and non-alcoholic liver disease.[2]
II. FACTORS TRIGGERING DYSBIOSIS AND DEGENERATIVE CHRONIC DISEASE
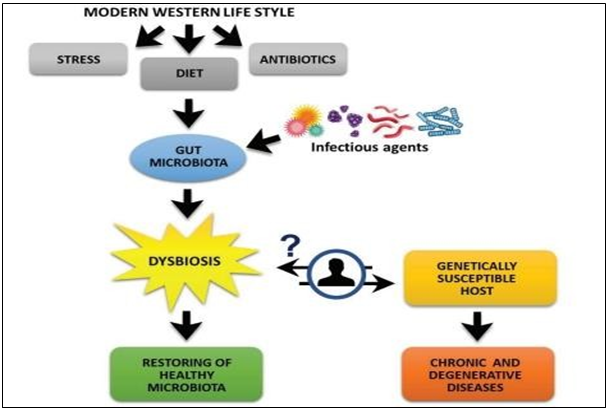
Figure 1: Factors triggering dysbiosis and degenerative chronic disease (Schippa & Conte, 2014)
III. INFLUENCE OF GUT MICROFLORA ON INFECTIOUS DISEASE
Numerous strategies have been identified by which the gut microbiome influences the emergence of infectious illnesses-
- Modulation of the immune system: The metabolites produced by the gut microbiome have the ability to affect the production of cytokines, chemokines, and other immunological mediators, hence modulating the immune system. The body's reaction to infections may be impacted by this.
- Barrier Function: To stop infections from moving from the lumen into the bloodstream, the mucus layer and the gut epithelium layer are essential. This barrier function may be compromised by an imbalance in the gut microbiota, which would allow infections to enter the bloodstream.
- Modulation of the epithelial barrier: The gut microbiome has the ability to produce metabolites that regulate the expression of tight junction proteins, which are essential for preserving the integrity of the epithelial barrier. This results in a modification of the epithelial barrier.
- Synthesis of antimicrobial peptides and Immune tolerance: Antimicrobial peptides produced by some gut bacteria aid in the defense against harmful microorganisms. By controlling immune response and lowering inflammation, the gut microbiota can enhance immunological tolerance. [1,2]
One of the gram-positive, obligate anaerobic, rod-shaped, spore-forming bacteria that makes up the human gut microbiota is C. difficile. Regular use of antibiotics disrupts the homeostasis of the intestinal mucosa, which lowers resistance to C. difficile toxin production and promotes the spread of Clostridium Difficile Infection (CDI). [3,4]
Helicobacter pylori, or H. pylori, is thought to be the cause of peptic disease. H. pylori is known to infiltrate the stomach mucosa and cause inflammation. Bacterial pathogens in tooth plaque and bacteria that cause gum disease in connection with H. pylori infection are also thought to be responsible, as they can accelerate the development of chronic periodontitis.[5,6]
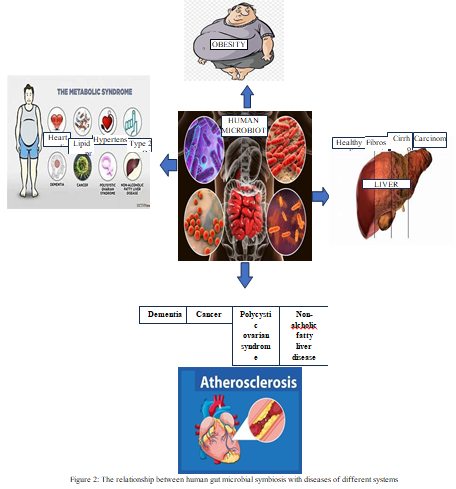
IV. THE CORRELATION BETWEEN MICROBIAL SYMBIOSIS IN THE HUMAN GUT AND MANY SYSTEMIC DISORDERS
V. NEUROPSYCHIATRIC DISORDERS-
1) Gut Microbiota’s Impact On Autism
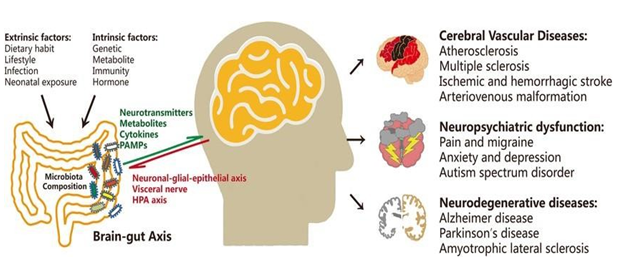
Figure 3: Neurological disorders and dysregulation of the gut microbiome. In addition to contributing to brain problems as cognitive impairment, dementia, and cerebrovascular diseases, extrinsic and intrinsic factors also influence the composition of the gut microbiota.
Behavioral stereotypes, impairments in cognition and social skills, and these are characteristics of neurodevelopmental conditions known as autism spectrum disorders (ASDs). The gut-brain axis, which is in charge of preserving healthy brain and gastrointestinal function, has been demonstrated to be significantly regulated by gut bacteria. Autism is caused by bacteria in the intestines.[8,21]
There is a negative link between Clostridium and Bifidobacteria in ASD patients because Clostridium produces propionate and exotoxins, which worsen ASD-like behavior. The gut microbial communities of children are unique and less varied, with much lower concentrations of Prevotella, Coprococcus, and unidentified Veillonellaceae. Studies on two types of bacteria: Desulfo vibrio species, which includes D. desulfuricans, and Bacteroides vulgates. D. piger and D. fairfieldensis- shown that they were found more frequently in the stools of children with AD than in the control group. Furthermore, compared to the control group, the overall flora of the stools of the AD children comprised less species of Firmicutes and Actinobacter.
2) Parkinson’s Disease
Parkinson's disease (PD) is a common neurodegenerative condition affecting the central nervous system. Its clinical hallmarks include abnormal a-synuclein aggregation in Lewy bodies and the loss and degeneration of dopaminergic neurons in the substantia nigra. An Numerous investigations showed that in the intestinal microbes of PD patients, the abundance of Bifidobacterium, Pasteurella, and Enterococcus increased dramatically while the abundance of Brauntella, Prevotella, and Faecococcus dropped. Parkinson's disease (PD) patients may have elevated Bifidobacterium as a defense against the worsening of neurodegenerative processes.
Patients with PD may also experience frequent constipation due to the increase of Lactobacillus, which has been shown to increase in Irritable Bowel Syndrome patients with constipation-like symptoms and decrease in those with diarrhea-like symptoms. The gut-brain axis is a two-way communication network that runs between the central nervous system and intestinal flora and is linked to the regulation of brain function, neural development, and aging. Between the gut microbiota and the brain axis, there are three main interactions.
Through SCFAs and other metabolites, bacteria can have an impact on the nervous system either directly or indirectly. influencing the neuroendocrine system and controlling GABA, 5-HT, and other neurotransmitter concentrations. The second is the brain pathways. The intestine and nonspecific neurological systems, which are impacted by flora and their metabolites, can have an effect on the brain and behavior. The last system is the immune system. Microglia and systemic cytokines (a measure of inflammation) are the two primary mediating mechanisms.
The pathophysiology of neurodegenerative disorders may involve several mechanisms.
Bacteria can affect the nervous system directly or indirectly in Parkinson's disease (PD) through chemical signals, neuronal pathways, or the immune system.
3) Alzheimer's disease
The most prevalent neurodegenerative condition affecting the central nervous system is Alzheimer's disease (AD). P-amyloid protein deposition and tau hyperphosphorylation in -amyloid plaques or neurofibrillary tangles are its defining features. These illnesses cause an inflammatory reaction that ultimately leads to irreparable brain damage and neuronal apoptosis or necrosis. Since both conditions start with a breakdown in the gut-brain axis, the pathophysiology of AD and Parkinson's disease have a comparable role for intestinal microbiota. Clinical studies indicate that gut flora contributes to the buildup of isoleucine, the control of neuroinflammation, and the improvement of cognitive impairment.
VI. THE MICROBIOTA-GUT BRAIN AXIS-
1) The relationship between obesity and intestinal flora
Health issues can arise from an imbalance between energy intake and expenditure. One such medical disease is obesity, which is defined as an abnormal or excessive accumulation of body fat. It has been linked to gut microbial dysbiosis, which may be species- or genus-specific. Conjugated linoleic acid (CLA) is an anti-obesity chemical that can be produced by some rumen bacteria. Short-chain fatty acids (SCFAs) are produced by gut bacteria from dietary fiber and can be utilized as a fuel source.[9,10,11]
2) The impact of gut microbiota on type 2 diabetes (T2D)
A wide range of human disorders, including type 1 and type 2 diabetes, have been linked to gut flora, as numerous studies have shown. Prevotella copri and Bacteroides vulgates are two instances of the modified microorganisms that induce inflammation by transferring microbiota from the gut to the tissues. It has been found that these microorganisms may potentially change the serum metabolome and lead to insulin resistance.[12,13]
3) Gut flora’s impact on Inflammatory Bowel Disease (IBD)
Crohn's disease and ulcerative colitis are the two main types of inflammatory bowel disease that primarily afflict young people and have a major negative impact on quality of life. Even though the precise origin of IBD is uncertain, it is widely accepted that environmental factors and genetically susceptible hosts' unintentional stimulation of the gastrointestinal tract immune system toward the gut microbiota is the reason.
Changes in the gut microbiota's diversity lead to an imbalance between pro- and anti-inflammatory microorganisms, which may have functional ramifications. For instance, it has been shown that the important member of the Firmicutes phylum, Faeca libacterium parasitizing, demonstrates anti-inflammatory qualities both in vitro and in vivo.[14]
4) Relationship between Type 1 diabetes (T1D) and gut microbiota
Insulin deficit is the result of the chronic autoimmune disease known as type 1 diabetes, which is defined by the death of the pancreatic beta cells that produce insulin. The autoimmune disease Type 1 diabetes is caused by the immune system destroying pancreatic β-cells.[15] Research has repeatedly demonstrated that the gut microbiota profiles of T1D patients differ from those of healthy people-
- Decreased bacterial species richness and diversity
- Overrepresentation of bacteria that could be harmful, like Streptococcus and Enterobacteriaceae
- Beneficial microorganisms like Lactobacillus and Bifidobacterium are underrepresented
5) The connection between gut flora and liver illness
The function of the gut microbiota is crucial for maintaining the gut-liver health communication. The gut microbiota produces substances including ethanol, ammonia, and acetaldehyde that are metabolized by the liver. It also controls the release of cytokines and the activity of Kupffer cells. Therefore, a major role for large-scale bacterial proliferation in the small intestine may play in the pathogenesis of non-alcoholic steatohepatitis (NASH). Increased intestinal permeability, both in terms of quantity and quality, as well as endotoxin transmission, can result from changes in the gut flora.[16]
Increased permeability of the gut lining can result from dysbiosis, or changes in the gut microbiome. permitting the passage of poisons and partially digested food particles to the liver. The production of pro-inflammatory substances by some gut bacteria can lead to liver inflammation and the development of diseases such as cirrhosis and non-alcoholic steatohepatitis (NASH).
The regulation of glucose and lipid metabolism is significantly influenced by the gut flora.
These processes can be interfered with by imbalances in the gut flora, which can result in metabolic illnesses such as type 2 diabetes and insulin resistance. The immune system is influenced by the gut microbiota, and changes to this microbiota can impact immune cell activity, resulting in chronic inflammation and hepatic damage.
Oxidative stress, which can harm the intestinal lining and change the makeup of the gut flora, can be caused by liver disease. Bile acids produced by the liver aid in the regulation of the gut microbiota. An imbalance in the production or absorption of bile acid might disrupt balance of intestinal flora.[17]
6) The relationship between between gut microflora and heart disease
Despite ongoing progress in treatment options, cardiovascular diseases (CVDs) continue to be the leading cause of death globally. It is believed that the gastrointestinal tract (GIT) experiences long-term translocation of microorganisms, which is normal and necessary for the GIT's microbial maturity in both healthy and diseased individuals. However, new studies have connected clinical issues with systemic inflammatory disorders to enhanced microbial translocation, and surgically treated patients have gained prominence in recent times.
DNA damage and an increased risk of gastric cancer can result from the production of genotoxic chemicals by some bacteria found in the gut microbiome. The development of gastric cancer can be aided by epigenetic alterations, such as DNA methylation and histone modification, that are influenced by the gut flora.[18, 19]
7) Gut microbiota and Pancreatic Cancer
It has been discovered that some bacteria, such Fusobacterium nucleatum, encourage the growth and spread of pancreatic tumors. Using probiotics or prebiotics to modify the gut flora may aid in the prevention or treatment of pancreatic cancer.[20]
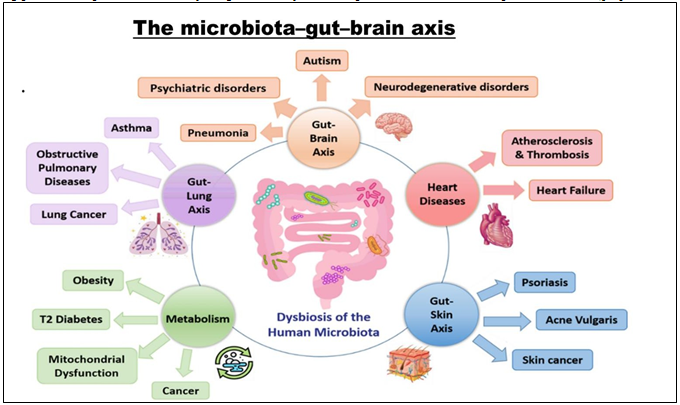
Figure 4: The human microbial dysbiosis in human disease. Gut microbiota is implicated in the right functioning of many organs, such as lungs, kidneys, liver, heart and brain. However, any disruption to the microbiota homeostasis results in the malfunctioning of these affected organs, and the progression of many related diseases.
VII. STRATEGIES TO MODIFY GUT MICROBIOMA FOR DISEASE TREATMENT-
Numerous physiological processes, including the onset of disease, are significantly influenced by the gut microbiota. Several diseases have been demonstrated to be effectively treated or prevented by altering the gut microbiome.[21,22]
- Probiotics- Probiotics are live bacteria that are given orally or via the rectum to alter the microbiota in the gut. Utilizing them can help to-Restore the gut microbiota's equilibrium, increase the generation of advantageous metabolites, reduce the signs of inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and other gastrointestinal diseases.
- Prebiotics- Prebiotics are non-digestible carbohydrates that can be given to the gut's beneficial microbes on a selective basis. They can be utilized to boost the synthesis of short-chain fatty acids and encourage the growth of beneficial microorganisms.[23]
- Synbiotics- Probiotics and prebiotics work together to produce a synergistic effect on the gut flora in synbiotics. They can be used to lessen the negative effects of probiotics and alleviate IBD and IBS symptoms. [24]
- Fecal Microbiota Transplantation (FMT)- It is the process of moving stool from a person in good health with a varied gut microbiota to a person in poor health. It is effective in treating Clostridium difficile infections and reestablishing a healthy gut microbiome.[25]
- Dietary changes and gut-directed nutrition- By feeding beneficial microbes into the gut, dietary modifications like increasing fiber intake can alter the gut microbiota. Similarly, gut-directed nutrition, which provides particular nutrients and supplements to support the growth of beneficial microbes in the gut, can improve immune system function and help manage weight.[26]
VIII. ADVANTAGES OF GUT FLORA FOR THE HOST-
- Depending on how the gut bacteria are spread and colonized, the host can benefit from them in different ways.
- Benefits include breaking down xenobiotic chemicals, converting bile acid and steroids, activating and removing toxins, genotoxins, and mutagens, as well as preserving and regulating gastrointestinal motility and vitamin production.
- In addition, a large amount of short-chain organic acids, such as butyric, propionic, and acetic acids, are produced in the colon's proximal region.
- The colonic mucosa and other tissues of the body use these organic acids as energy sources. They are byproducts of complex carbohydrates that have not been fully digested and are fermented by colonic bacteria.
- By lowering faecal pH and changing colonic water absorption, these organic acids have a direct effect on colonic bacterial growth.
- The breakdown and digestion of indigestible materials, the supply of essential nutrients, protection against opportunistic pathogen invasion, and assistance in the development of intestinal architecture are just a few of the numerous roles played by gut symbiotic bacteria.[27] For example, intestinal microbiota aids in maintaining energy balance by breaking down food particles that have been left undigested in the stomach and small intestine.
- Certain species of Bacteroides break down foods containing fiber, primarily dietary fibers like xyloglucans, which are commonly found in vegetables.
- Both Lactobacillus and Bifidobacterium use other nondigestible fibers, such as fructo-oligosaccharides and oligosaccharides.
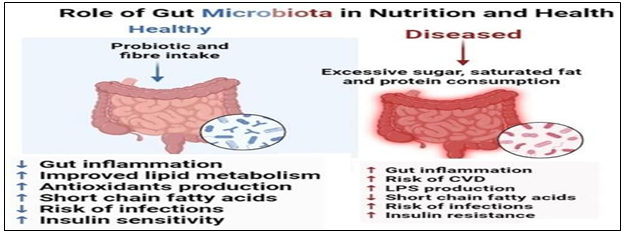
Figure 5: Diagram showing the microbiome in health and dysbiosis
Conclusion
This review\'s objective is to present the most recent research on the relationship between gut flora and human health and disease. Numerous illnesses, including as IBD, obesity, diabetes, cancer, HIV, and autism, are brought on by the dysbiosis of bacteroides in the gut. To precisely understand the pathophysiology and role that gut bacteria play in disease, further research needs to be done. It has been shown that butyrate is a very important vitamin for the upkeep of healthy colon cells, suppression of growth and induction of apoptosis in human colon cancer. Future research should focus on identifying the specific pathways through which the gut microbiota affects immunological, neurological, and metabolic processes. It is also necessary to investigate the benefits of combining probiotics and prebiotics for human health. More research is required to identify the bacteria that produce butyrate. The advancement of metagenomics, metabolomics, and other omics technologies will allow for a deeper understanding of these interactions. Methods like microbiome characterization used in personalized medicine may lead to the development of specialized medicines for the prevention and treatment of various health conditions. Furthermore, research into the therapeutic potential of fecal microbiota transplantation probiotics and prebiotics may provide fresh approaches to modifying the gut microbiome and enhancing general health. It will need multidisciplinary studies integrating immunology, neurology, computational biology, and microbiology to completely understand how the gut microbiome affects human health and disease ,paving the way for innovative therapeutic strategies.
References
[1] Admassie M. Assefa F, Alemu T. Gut Microbiota on Human Health, Discase and Attainment of the Human Gut Microbiota, Appli Microbiol Open Access, 2020, Vol.6 Iss.3 No: 179. [2] Wang B. Yao M. Lv L. Ling Z, Li L. The human microbiota in health and disease, Engineering, 2017: 3: 71-82 [3] Hu Z, Zhang Y, Li Z. Effect of Helicobacter pylori infection on chronic periodontitis by the change of microccology and inflammation, Oncotarget, 2016; 7:66700 [4] Gorobchenko K. Effects of probiotics on intestinal microflora of HIV infected individuals, J Advances in Microbiol, 2017: 1-7. [5] Zhang YJ. Li S. Gan RY, Zhou T. Xu DP, Li HB. Impacts of gut bacteria on human health and diseases. International J Molecular Sci., 2015; 16: 7493-7519 [6] Ramakrishna B S, Role of the gut microbiota in human nutrition and metabolism, Journal of Gastroentology and Hepatology,2013, doi: 10.1111/jgh.12294 [7] Marzal P. L.N. Prince, N.; Bajie D.; Roussin, L. Naudon, L Rabot, S Garssen, J Kraneveld, Pardo P, P. The Impact of Gut Microbiota-Derived Metabolites in Autism SpectrumDisorders. Int. J. Mol. Sci. 2021, 22,10052. https://doi.org/10.3390/ims221810052 [8] Sherwin, E., Bordenstein, S.R., Quinn, J.L, Dinan, T.G., Cryan, J.F. Microbiota and the social brain. Science 2019, 366, 2016. [CrossRef] [PubMed] [9] Haas S., Haghikia A, Wilck N, Muller D.N., Linker R.A. Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology 2018, 154, 230-238.[CrossRef) [10] Vadder D, Grasset F, Holm E M, Karsenty L, Macpherson G, Olofsson A J, Bäckhed LE. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proe. Natl. Acad. Sci. USA 2018, 115, 6458-6463.[CrossRef] [PubMed) [11] Prince L, Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [CrossRef] [12] Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell, 2016,167, 915-932. [CrossRef] [13] Li, Q.; Zhou, J.M. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience, 2016,324, 131-139. [CrossRef] [14] Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems, Ann. Gastroenterol,2015, 28, 203-209. [PubMed] [15] Serra D & Leonor M, Almeida & Teresa, Dinis C P, The Impact of Chronic Intestinal Inflammation on Brain Disorders:the Microbiota-Gut-Brain Axis, Molecular Neurobiology, 2019, https://doi.org/10.1007/s12035-019-1572-8 [16] Fetarayanil D. Hariyono H, Soegiarto G, The role of gut microbiota in health and diseases. Qanun Medika. 2021. vol 5 [17] Ley. R.E., Turnbaugh, P.J.. Klein, S., & Gordon, J.I. The human gut microbiome:Ecology and recent evolutionary changes, Nature, 444(7122).2006, 1022-1023. doi: 10.1038/nature05414 [18] Cryan, J.F., & Dinan, T.G., Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience,2012, 13(10), 701-712. doi: 10.1038n3346 [19] Rastogi S. Mohanty S, Sharma S, Tripathi P. Possible role of gut microbes and host\'s immune response in gut lung homeostats is, Frontiers in immunology,2022, vol. 13 [20] Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, MerluzziAP, Johnson SC, Carlsson CM, Asthana S et al, Gut microbiome alterations in Alzheimer\'s disease,2017, Sci Rep 7:13537-13548, doi: 10.1038/41598-017-13601-y. [PubMed] [21] Albenberg LG, Wu GD, Diet and the intestinal microbiome: associations, functions, and implications for health and disease, Gastroenterology, 2014, 146:1564-1572 [22] Zeng MY, Inohara N, Nunez G, Mechanisms of inflammation-driven bacterial dysbiosis in the gut, Mucosal Immunol ,2017, 10:18-26 [23] Houser MC, Tansey MG, The gut-brain axis: is intestinal inflammation a silent driver of Parkinson\'s disease pathogenesis, NPJ Parkinsons Dis, National Library Of Medicine, 2017, 11:3:3, doi: 10.1038/s41531-016-0002-0 3:3-12[PubMed] [24] Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP, Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders, Front Cell Neurosci,2015,9:392 412,doi:10.3389/fincel.2015.00392., [PubMed] [25] Sharma S, Tripathi P, Gut microbiome and type 2 diabetes: where we are and where to go? The Journal of nutritional biochemistry,2019, vol.63, page 101-108 [26] Panwar S, Sharma S, Tripathi P, Role of barrier integrity and dysfunctions in maintaining the healthy gut and their health outcomes, Frontiers in Physiology,2021,vol.12
Copyright
Copyright © 2024 Ayushi Maurya, Sankarasubramanian Vidyameenakshi , Neerja Masih. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64782
Publish Date : 2024-10-24
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

