Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Tranexamic Acid and its Role in Total Knee Replacement
Authors: Mr. A. Prathap, Roja M K, Samyuktha D V, Saranya R, Shruthi D, Priyadarshini P
DOI Link: https://doi.org/10.22214/ijraset.2023.55659
Certificate: View Certificate
Abstract
Tranexamic acid is a synthetic lysine derivative that inhibits the lysine binding sites on plasminogen molecules in order to have an antifibrinolytic action. In patients with upper gastrointestinal bleeding, tranexamic acid was related to mortality decreases of 5 to 54% when compared to placebo. A 40% reduction was found by meta-analysis. Tranexamic acid therapy reduced mean menstrual blood loss in menstruating women with menorrhagia by 34 to 57.9% in comparison to placebo or control; the medication has also been successfully used to treat placental bleeding, postpartum hemorrhage, and cervix conization. In hemophiliac patients undergoing oral surgery, tranexamic acid considerably decreased mean blood losses, and it worked well as a mouthwash for dental patients taking oral anticoagulants. With the administration of the medication, blood loss was also decreased in patients having orthotopic liver transplantation or transurethral prostate surgery, and rebleeding rates were decreased in patients with traumatic hyphaema. Patients with hereditary angioneurotic edema have also claimed clinical improvement from tranexamic acid. The most frequent adverse reactions to tranexamic acid are nausea and diarrhea. Clinical trials have not shown that the medication increases the risk of thrombosis.
Introduction
I. INTRODUCTION
The blood coagulation cascade, triggered when a blood artery is damaged, produces thrombin, which degrades soluble fibrinogen into minute peptides to produce fibrin monomers. Following the polymerization of these monomers, fibrin is created, which is joined by noncovalent bonds. The activation of transamidase factor XIIIa by thrombin results in the formation of covalent bonds between fibrin molecules and the formation of a clot that is resistant to dissolution. Together with aggregated platelets, insoluble fibrin aggregates block the damaged blood artery and stop further bleeding. Yet, a balance between the production and lysis of fibrin must exist to maintain and reshape the hemostatic barrier throughout the few days it takes to mend the injured vessel wall.1
Major morbidity and mortality are linked to blood loss and subsequent transfusions. By using antifibrinolytics, blood loss during cardiac surgery, trauma, orthopedic surgery, liver surgery, solid organ transplantation, obstetrics and gynecology, neurosurgery, and non-surgical disorders can be reduced. 2,3 In order to reduce bleeding in various circumstances, the fibrinolytic inhibitor tranexamic acid (TA) was created in the 1960s and quickly used in therapeutic settings. The overwhelming data demonstrates that this medication is helpful in reducing blood loss, reducing the need for transfusions, and not increasing the likelihood of vascular occlusive events. The best dosage, the danger of thrombosis and other adverse effects, as well as potential new indications for use, are still up for debate. The TA is the same old substance that is currently the focus of continuing international randomized trials in patients with various conditions, such as severe head injuries and severe postpartum bleeding. In patients being treated with inhibitors of platelet function and the new oral anticoagulant medications, which are now being administered to patients in a growing number of cases, TA may also play a significant role in limiting bleeding.
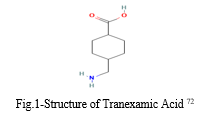
A monocarboxylic acid is a tranexamic acid. It functions as a hematologic agent and an antifibrinolytic medication. It is similar to cyclo-hexane carboxylic acid in terms of function.6 (Fig.1)
A. Pharmacodynamic
Tranexamic acid forms a reversible compound with plasminogen that has an antifibrinolytic action. Lysine binding sites on human plasminogen are crucial for interactions with fibrin, 2-antiplasmin, and fibrinogen in addition to synthetic antifibrinolytic amino acid derivatives.4 Merely one of these binding sites has a high affinity for tranexamic acid (Kd = 1.1 mol/L), whereas the rest only have a low affinity (Kd = 750 mol/L). Tranexamic acid, principally by binding to the high-affinity lysine binding site of plasminogen, virtually totally prevents the interaction of plasminogen and the heavy chain of plasmin with the lysine residues of fibrin monomer.5 Plasminogen cannot bind to the surface of fibrin when this location is saturated with tranexamic acid. (Fig.2)

Tranexamic acid inhibits trypsinogen activation by enterokinase in a competitive manner, and at doses four times higher, it inhibits trypsin's proteolytic activity in a non-competitive manner. The medication also hardly suppresses thrombin.7 The medication also hardly suppresses thrombin.8 The lysine derivative TA, which has a molecular weight of 157, blocks the lysine-binding sites on the plasminogen molecules in order to indirectly exert its antifibrinolytic activity.
B. Pharmacokinetic
The peak plasma concentration after IV administration of TA 10 mg/kg was attained after an hour of injection, according to pharmacokinetic investigations in healthy adults. About 80 minutes was believed to be the biological half-life. 90% of the prescribed dose was eliminated in the urine after 24 hours, compared to 30% after the first hour. The highest plasma concentration was attained in 3 hours following oral dosages of 10 to 15 mg/kg.9 Gastrointestinal adsorption was unaffected by food.10 TA builds up in the tissues11 and quickly permeates joint fluids and synovial membranes.12 Even though the quantities in breast milk are 100 times lower than those in plasma, it crosses the placenta to reach the developing fetus.13,14 In toxicological investigations, no teratogenic effects were identified.14 Healthy participants who had taken a single dose of 1g of tranexamic acid orally had no tranexamic acid found in their saliva.15 However, after mouth washing for 2 minutes with a 5% aqueous solution of tranexamic acid, very high drug concentrations (mean 200 mg/L) were reached in saliva 30 minutes later, whereas plasma concentrations stayed below 2 mg/L.
C. Tolerability
Adverse reactions to tranexamic acid medication are rare; nausea, diarrhea, and rarely orthostatic reactions are the most frequently reported side effects.16 A rare case report of cerebral thrombosis, 17,18 arterial thrombosis,19 severe renal failure,20,21 and coronary graft occlusion22 in patients taking tranexamic acid highlights the theoretical danger of increased thrombotic tendency during therapy with inhibitors of fibrinolysis. A retrospective review of the case files of 256 pregnant women with bleeding issues, 168 of whom underwent cesarean sections, revealed no evidence of tranexamic acid's thrombogenic effects.23 These findings are especially important because pregnant women have a reduced ability to break down fibrin and an increased risk of thrombosis (especially following a cesarean section). Furthermore, no retinal alterations were observed in patients who took the medication at therapeutic dosages for intervals ranging from 15 months to 8 years.24 However, it is advised that any patients who experience this symptom stop taking tranexamic acid as soon as possible. This is because cases of color vision disturbance have been documented.25
II. DOSAGE AND ADMINISTRATION
For intravenous usage, tranexamic acid is offered in 500 mg/5 ml ampoules, 500 mg tablets, and syrup in a 5 ml size. It is advised to take 500 mg to 1 g by slow intravenous injection three times per day for local fibrinolysis, or 1 to 1.5 g orally two to three times per day. An intravenous injection given slowly at a dose of 1g or 10 mg/kg is advised for general fibrinolysis. For youngsters, dosages should be based on body weight at a rate of 25 mg/kg/dose.65 Other dosages for specific indications are as follows:
- Tranexamic acid has typically been administered intravenously to patients having cardiac surgery as a 10 mg/kg dose prior to CPB and a 1 mg/kg/hour infusion afterward.66 The most often utilized dosage in patients with upper GI bleeding has been 4.5 to 6g daily (split into 3 to 6 doses) for 5 to 7 days (intravenous followed by oral therapy).
- A 4.8 to 5% mouthwash, administered for two minutes four times per day for seven days, has shown good efficacy in dental patients undergoing anticoagulant medication. Patients with hemophilia who are scheduled to have oral surgery need 1 to 1.5g orally every eight hours.
- Patients having knee arthroplasty may get an intravenous infusion of 10 mg/kg before the relaxation of the tourniquet, while patients having transurethral prostate surgery may receive oral tranexamic acid therapy for 4 days at a dose of 6 to 12 g per day. Patients receiving an orthotopic liver transplant have had success using an intravenous infusion of 40 mg/kg/hour.
- For 3 to 4 days, tranexamic acid 1 to 1.5g should be given orally to menstruating women. For conization of the cervix or traumatic hyphaema, dosages of 1.5g or 1 to 1.5g orally three times daily are indicated, respectively. For the management of hereditary angioneurotic edema, oral treatment with 1.5g three times daily is advised.
- Those with a history of the thromboembolic disease should not use tranexamic acid, and individuals with renal impairment should take it at lower doses.65
III. ADVERSE EFFECTS
- Generalized Fixed Drug Eruption: A particular kind of cutaneous drug reaction known as a fixed drug eruption (FDE) occurs in the same areas after repeated exposure to the offending medication. It manifests as a single or few red or violaceous plaques that disappear and are followed by post-inflammatory hyperpigmentation. Women between the ages of 27 and 39 have presented all three cases of fixed eruption after taking TXA. In one instance, after taking TXA-containing medications, a Japanese woman had previously gone through repeated episodes of erythema and postinflammatory pigmentation on the back.68
- Epidermal Necrolysis: Pretel et al. documented a case of a 67-year-old man who received TXA 1000 mg every 8 hours for acute rectal bleeding. Ten days into TXA therapy, the patient's trunk developed a reddish macular rash. The lesions grew larger during the course of the following days and confluence with blisters and epidermal necrosis spread to several mucosal surfaces. Following the healing of the skin lesions, TXA was stopped and prednisone therapy was initiated. Skin biopsy results supported the toxic epidermal necrolysis diagnosis. Nevertheless, the patient died within two weeks due to acute renal failure, a respiratory infection, and multiorgan failure despite the benign remission of TEN.69
- Ligneous Conjunctivitis: Recently, Song et al. reported a case of ligneous conjunctivitis caused by TXA with reversible hypoplasminogenaemia. Oral TXA (750 mg/day) was used to treat a stomach ulcer in a 70-year-old female patient. She had a bilateral pale yellow pseudomembrane on the palpebral conjunctivae after receiving therapy for 5 weeks. Hypoplasminogenaemia was detected by a hematological analysis. A diagnosis of ligneous conjunctivitis was made, and daily topical cyclosporine treatment was started without much success. The pseudomembranes retreated and the serum plasminogen level restored to the normal range when TXA was stopped. Since the past has convincingly established the link between hypoplasminogenaemia and ligneous conjunctivitis. 1 and in the second report, TXA was stopped, and the hypoplasminogenaemia improved.70
- Ventricular Dysfunction: Samanta et al. found that a polytrauma patient who received TXA injections experienced wide left and right ventricular dysfunction. A 45-year-old polytrauma patient who had been bleeding profusely from external wounds developed an adverse reaction characterized by dyspnea, chest tightness, palpitations, tachycardia, itching in the left hand, and developed rashes (urticaria) over his upper limb and chest within 20 minutes of receiving a TXA 1g injection. A little bronchospasm was observed on auscultation. A substantial ST depression was evident on a second ECG. Serial cardiac enzymes were within normal limits at 2, 6, and 12 hours. At the second and twelve hours, transthoracic echocardiography examination indicated worldwide left and right ventricular hypokinesia. The next day's coronary angiography revealed no obvious coronary artery disease.71
IV. CONTRAINDICATIONS
A history of venous or arterial thromboembolism, cerebral bleeding, known color vision defects, a known allergy to TXA, or thromboembolic illness that is currently active. More than three hours following a serious injury.67
A. Caution
TXA is not well studied in the renally impaired. It is 95% excreted in the urine, so renal dosing is recommended, and judicious administration in patients with severe renal impairment.
No adjustments are required in the hepatic impaired patient. 67
B. Pregnancy Category
Pregnancy category B applies to TXA. Animal studies show no harm or very low risk, while human studies show no risk.67
C. Breastfeeding
Infants who were breastfed while being exposed to tranexamic acid did not experience any worsening of their long-term results. Our findings in conjunction with earlier predictions of extremely minimal medication exposure encourage women who need tranexamic acid treatment to continue nursing.67
D. Total Knee Replacement
In knee replacement surgery, sometimes referred to as arthroplasty, diseased knee components are removed and replaced with prosthetic devices, or prostheses.
Your orthopedic joint replacement surgeon will remove the diseased and damaged ends of the femur (thigh bone) and the top of the tibia (shin bone) and replace them with artificial implants made of metal or plastic. Each bone has a perfect fit for the prosthetic. After that, parts are frequently attached to the thigh, shin, and kneecap using surgical cement. An operation to resurface the patella, the back of the kneecap, may be necessary.41
The three components of the artificial prosthesis are as follows:
- Femoral Component: A metal cap that fits over the femur's end and features a groove that lets the kneecap move up and down as the knee bends and straightens
- Tibial Component: A flat piece of metal with a plastic plate covering the top of the tibia.
- Patellar Component: A dome-shaped piece of plastic that permits it to glide.41
Although arthritic pain might start suddenly, it often develops over time and gradually. You can experience pain when getting up the first time in the morning or after sitting for a while. Several people assert that variations in weather might make arthritis discomfort worse. At some point, you could realize that the discomfort is making it difficult for you to perform your everyday tasks. Even sitting down could hurt. So could walking and climbing stairs. It's possible that you can't bend or straighten your knee as well as you used to. Moreover, you can feel your knee grind and hear popping and crackling noises while walking. Even in your sleep, arthritis may cause you to awaken. Your knee's degeneration from the joint disintegration could alter and deform its appearance.
After exhausting all available conservative remedies with no success, knee replacement surgery is recommended. Consider making a decision that will enable you to reclaim your life if you have tried every prescription your doctor has prescribed but are still struggling with pain, stiffness, or loss of stability. Knee replacement surgery may be the only available option in the future.41
Whole knee arthroplasty is one of the most common surgical procedures in the United States (TKA).26 The reconstruction of the knee joint is known as knee arthroplasty. It is more frequently referred to as a total knee replacement and is a procedure with generally reliable results.
Total knee arthroplasty (TKA) is a fantastic choice for persons with symptomatic osteoarthritis in at least two of the three compartments of the knee and who have not responded to conservative treatment.27,28 The only therapy that effectively lessens chronic knee pain (TKR) is total knee replacement.29 TKR is frequently regarded as an effective and successful end-stage surgical surgery for addressing persistent knee pain and functional disability based on results from surgeon-based outcome tools and survivorship analyses. 30,31 TKR is frequently regarded as an effective and successful end-stage surgical surgery for addressing persistent knee pain and functional disability, based on results from surgeon-based outcome tools and survivorship analyses. 32,33
The most common cause of a primary knee replacement, or TKA, is osteoarthritis. 32,33 Osteoarthritis wears down the joint cartilage, rendering it unable to absorb shock. Risk factors for osteoarthritis of the knee include gender, a higher BMI, a history of knee traumas, and comorbidities. 34,35 A total knee replacement is more common in women and those over the age of 50.36,37In the US and the UK, women were the majority of patients for TKA treatments.36,38 There will be a significant rise in TKA procedures due to the anticipated trend of younger TKA recipients, or those under the age of 60.38,40 Younger patients receiving TKA for knee osteoarthritis are more likely to be morbidly obese, smoke, and hope to resume physically demanding activities like sports, according to Hawker et al.39
V. ROLE OF TRANEXAMIC ACID IN TOTAL KNEE REPLACEMENT
With blood loss ranging from 800 ml to 1800 ml after elective total knee arthroplasty (TKA), postoperative bleeding is still one of the key concerns. A tourniquet may increase the fibrinolysis brought on by surgical trauma, which lowers the risk of venous thromboembolism but favors postoperative blood loss. Many strategies have been investigated to lower postoperative blood loss, including recombinant human erythropoietin usage, purposeful hypotension, perioperative blood donation, and perioperative red cell salvage.42-44 Perioperative transfusions also raise the cost of care and increase the patient's risks (such as those of infection, allergy, and disease transfer, for example).45,46 Recently, pharmacological techniques have become more prominent. Since hyperfibrinolysis is regarded to be the main cause of postoperative bleeding after TKA surgery, antifibrinolytic drugs such as aprotin, aminocaproic acid, and tranexamic acid have been recommended (tXA). By blocking the proteolytic activity of the plasminogen activator, the fibrinolysis inhibitor tXA prevents clot lysis.47 The intravenous (IV) administration of tXA is thought to enhance the risk of thrombotic events. Moreover, there have been some instances of allergic responses to tXA. Because of these concerns, some experts have come to the conclusion that tXA should not be administered to patients who have a history of allergies, arterial or venous thrombosis, an intrinsic risk of thrombosis or thromboembolism, acute renal failure, subarachnoid hemorrhage, or epilepsy.48 Further research has shown that the common postoperative prophylactic regimens against deep vein thrombosis (DVT), including aspirin, warfarin, low-molecular-weight heparin (LMWH), and even factor Xa inhibitor, can prevent the alleged increased risk of thrombotic events.49-52 Several publications have recommended topical IA injection of tXA prior to wound closure to reduce the risk of problems brought on by thrombotic events.44,53,54
Although the reported doses of IV TXA ranged from 10 to 20 mg/kg, a 1 g dose (between 500 mg and 3 g) was frequently used in studies. From 2 mg/kg/h for 20 hours to 10 mg/kg/h for three hours, the dosages for continuous infusions range. IV TXA has also been given as a single dose bolus and as a continuous infusion for two to three hours.55-57 TXA doses range from 250 mg to 3 g when administered topically, and they are dissolved in saline solutions ranging in volume from 75 to 250 ml. The effectiveness of tXA when combined with other topical treatments, such as povidone-iodine solution (3 g of topical tXA in povidone-iodine solution), has also been demonstrated.58
According to earlier studies, which established the therapeutic plasma concentrations of tranexamic acid at 10 ng ml-1 16, an 80% decrease in the activity of plasminogen activator is necessary for the inhibition of fibrinolysis in tissues.60,61 An intravenous dose of 10 mg kg-1 tranexamic acid only maintains such plasma levels for three hours.62 Since it might not be enough to control postoperative bleeding in prosthetic knee surgery, there is a case for using higher doses.59
VI. DISCUSSION
Surgery for a total knee replacement must take blood loss into consideration because it is a severe problem. The usage of tranexamic acid appears to be simpler, more affordable, and problem-free compared to other techniques of reducing blood loss. Surgery-related trauma activates both the intrinsic and extrinsic coagulation pathways. The body has a typical fibrinolytic pathway that is simultaneously triggered in order to manage uncontrolled coagulation. By breaking down the fibrin in the clot, the pathway's final product, plasmin, tips the scales in favor of clot disintegration.63
As an antifibrinolytic, tranexamic acid prevents the production of active plasmin, hence preventing the breakdown of clots.63 So, using this medication lessens bleeding while hypothetically causing deep vein thrombosis.64
Conclusion
Numerous hemorrhagic diseases benefit from tranexamic acid treatment. In addition to potentially being more affordable and tolerable than aprotinin, the medication appears to lower mortality rates and the need for urgent surgery in patients with upper gastrointestinal hemorrhage. It also reduces postoperative blood losses and the need for transfusions in a variety of surgical procedures. In addition to successfully controlling bleeding during pregnancy, tranexamic acid also lessens menstrual blood loss and may be used instead of surgery to treat menorrhagia. TXA administration should be based on clinical judgment, informed by patient history, thromboelastometry, laboratory, and radiologic investigation, and adapted to the treatment location and capability for intervention and transfusion.
References
[1] Merck & Co. I. Hematology and Oncology: hemostasis and Cardiovasc Surg coagulation disorders. [2] Karkouti K, Wijeysundera DN, Yau TM et al.: The independent association of massive blood loss with mortality in cardiac surgery. Transfusion 2004; 44: 1453–1462. [3] Karkouti K, Beattie WS, Dattilo KM, et al.: A propensity score case-control comparison of aprotinin and tranexamic acid in high-transfusion-risk cardiac surgery. Transfusion 2006; 46: 327–338. [4] Thorsen S. Differences in the binding to fibrin of native plasminogen and plasminogen modified by proteolytic degradation: influence of?-amino carboxylic acids. Biochim Biophys Acta 1975; 393: 55-65 [5] Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta 1981 Feb 18; 673: 75-85 [6] National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 5526, Tranexamic acid. Retrieved February 14, 2023. [7] Dubber AH, McNicol GP, Douglas AS. Amino methyl cyclo-hexane carboxylic acid (AMCHA), a new synthetic fibrinolytic inhibitor. Br J Haematol 1965; 11: 237-45 [8] Andersson L, Nilsson IM, Niléhn JE, et al. Experimental and clinical studies on AMCA, the antifibrinolytic ally active isomer of p-aminomethyl cyclohexane carboxylic acid. Scand J Haematol 1965; 2: 230-47 [9] Eriksson O, Kjellman H, Schannong M. The biological availability of Cyklokapron tablets compared with Cyklokapron solution administered orally. Stockholm, Sweden: Kabi AB; 1971. [10] Pilbrant A, Schannong M, Vessman J. Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol 1981;20:65–72. [11] Andersson L, Nilsson IM, Colleen S, Granstrand B, Melander B. Role of urokinase and tissue activator in sustaining bleeding and the management thereof with EACA and AMCA. Ann N Y Acad Sci 1968;146:642–58. [12] Ahlberg A, Eriksson O, Kjellman H. Diffusion of tranexamic acid to the joint. Acta Orthop Scand 1976;47:486–8. [13] Kullander S, Nilsson IM. Human placental transfer of an antifibrinolytic agent (AMCA). Acta Obstet Gynecol Scand 1970;49:241–2. [14] Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol) 1980;14:41–7. [15] Sindet-Pedersen S. Distribution of tranexamic acid to plasma and saliva after oral administration and mouth rinsing: a pharmacokinetic study. J Clin Pharmacol 1987 Dec; 27: 1005 [16] Verstraete M. Clinical application of inhibitors of fibrinolysis. Drugs 1985 Mar; 29: 236-61 [17] Fodstad H, Liliequist B. Spontaneous thrombosis of ruptured intracranial aneurysms during treatment with tranexamic acid(AMCA): report of three cases. Acta Neurochir Wien 1979; 49: 129-44 [18] Rydin E, Lundberg PO. Tranexamic acid and intracranial thrombosis [letter]. Lancet 1976 Jul 3; II: 49 [19] Davies D, Howell DA. Tranexamic acid and arterial thrombosis [letter]. Lancet 1977 Jan 1; I: 49 [20] Albronda T, Gökemeyer JDM, van Haeften TW. Transient acute renal failure due to tranexamic acid therapy for diffuse intravascular coagulation. Neth J Med 1991 Aug; 39: 127-8 [21] Fernández Lucas M, Liaño F, Navarro JF, et al. Acute renal failure secondary to antifibrinolytic therapy. Nephron 1995 Apr; 69: 478-9 [22] Robblee J. Graft occlusion following administration of tranexamic acid. Anesth Analg 1995 Apr; 80 Suppl.: SCA141 [23] Lindoff C, Rybo G, Åstedt B. Treatment with tranexamic acid during pregnancy, and the risk of thrombo-embolic complications. Thromb Haemost 1993 Aug 2; 70: 238-40 [24] Theil PL. Ophthalmological examination of patients in long-term treatment with tranexamic acid. Acta Ophthalmol Copenh 1981 Apr; 59: 237-41 [25] Pharmacia & Upjohn. Cyklokapron. ABPI Compendium of Data Sheets and Summaries of Product characteristics 1998- 1999. London: Datapharm Publications Ltd [26] Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227–1236. [27] Davies PS, Graham SM, Maqungo S, Harrison WJ. Total joint replacement in sub-Saharan Africa: a systematic review. Trop Doct. 2019 Apr;49(2):120-128. [28] Adie S, Harris I, Chuan A, Lewis P, Naylor JM. Selecting and optimizing patients for total knee arthroplasty. Med J Aust. 2019 Feb;210(3):135-141. [29] Juni P, Reichenbach S, Dieppe P. Osteoarthritis: a rational approach to treating the individual. Best Pract Res Clin Rheumatol 2006;20:721–40. [30] Ewald FC, Wright RJ, Poss R, Thomas WH, Mason MD, Sledge CB. Kinematic total knee arthroplasty: a 10- to 14-year prospective followup review. J Arthroplast 1999;14:473–80. [31] Anderson JG, Wixson RL, Tsai D, Stulberg SD, Chang RW. Functional outcome and patient satisfaction in total knee patients over the age of 75. J Arthroplast 1996;11:831–40 [32] Evans JT, Walker RW, Evans JP, Blom AW, Sayers A, Whitehouse MR. How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. The Lancet. 2019 Feb 16;393(10172):655-63. [33] Meena A, Hoser C, Abermann E, Hepperger C, Raj A, Fink C. Total knee arthroplasty improves sports activity and the patient-reported functional outcome at mid-term follow-up. Knee Surgery, Sports Traumatology, Arthroscopy. 2022 Jun 11:1-9. [34] Blagojevic, M., Jinks, C., Jeffery, A. and Jordan, K. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis and Cartilage. 2010;18(1):24 [35] Driban J, McAlindon T, Amin M, Price L, Eaton C, Davis J et al. Risk factors can classify individuals who develop accelerated knee osteoarthritis: Data from the osteoarthritis initiative. Journal of Orthopaedic Research. 2017;36(3):876-880. [36] Medscape. Total knee arthroplasty (TKA). Available from: https://emedicine.medscape.com/article/1250275-overview#:~:text=The%20primary%20indication%20for%20total,pain%20caused%20by%20severe%20arthritis. [37] Knee Replacement Surgery By The Numbers - The Center [Internet]. The Center Orthopedic and Neurosurgical Care & Research. 2020 [cited 22 December 2020]. [38] Singh J, Yu S, Chen L, Cleveland J. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. The Journal of Rheumatology. 2019;46(9):1134-1140. [39] Hawker GA, Bohm E, Dunbar MJ, Jones CA, Noseworthy T. The Effect of Patient Age and Surgical Appropriateness and Their Influence on Surgeon Recommendations for Primary TKA: A Cross-Sectional Study of 2,037 Patients. JBJS. 2022 Apr 20;104(8):700-8. [40] Ravi B, Croxford R, Reichmann W, Losina E, Katz J, Hawker G. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Practice & Research Clinical Rheumatology. 2012;26(5):637-647. [41] https://www.merillife.com/patients-caregivers/orthopedics/total-knee-replacement-surgery [42] Cushner FD, Friedman RJ. Blood loss in total knee arthroplasty. Clin orthop Relat Res. 1991;(269):98-101. [43] sehat KR, Evans R, newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. 2000;7:151- 155. [44] soni A, saini R, Gulati A, et al. Comparison between intravenous and intra-articular regimens of tranexamic acid in reducing blood loss during total knee arthroplasty. J Arthroplasty. 2014;29:1525-1527. [45] Bierbaum BE, Callaghan JJ, Galante Jo, et al. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint surg Am. 1999;81:2-10. [46] Forbes JM, Anderson MD, Anderson GF, et al. Blood transfusion costs: a multicenter study. transfusion. 1991;31:318- 323 [47] Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad orthop surg. 2010;18:132-138 [48] Tengborn L, Blombäck M, Berntorp E. tranexamic acid-an old drug still going strong and making a revival. thromb Res. 2015;135:231-242 [49] Poeran J, Rasul R, suzuki s, et al. tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United states: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829. [50] Gillette BP, Desimone LJ, trousdale Rt, et al. Low risk of thromboembolic complications with tranexamic acid after primary total hip and knee arthroplasty. Clin orthop Relat Res. 2013;471:150-154. [51] Onodera t, Majima t, sawaguchi n, et al. Risk of deep venous thrombosis in drain clamping with tranexamic acid and carbazochrome sodium sulfonate hydrate in total knee arthroplasty. J Arthroplasty. 2012;27:105-108. [52] Tan J, Chen H, Liu Q, et al. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J surg Res. 2013;184:880-887 [53] 16. Patel Jn, spanyer JM, smith Ls, et al. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014; 29:1528-1531. [54] Chen s, Wu K, Kong G, et al. the efficacy of topical tranexamic acid in total hip arthroplasty: a meta-analysis. BMC Musculoskelet Disord. 2016;17:81 [55] Aggarwal AK, Singh n, Sudesh P. topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016; 31:1442-1448. [56] Lin ZX, Woolf sK. safety, efficacy, and cost-effectiveness of tranexamic acid in orthopedic surgery. orthopedics. 2016; 39:119-130. [57] Keyhani s, Esmailiejah AA, Abbasian MR, et al. Which route of tranexamic acid administration is more effective to reduce blood loss following total knee arthroplasty? Arch Bone Jt Surg. 2016;4:65-69. [58] Carvalho LH Jr, Frois temponi E, Machado soares LF, et al. Bleeding reduction after topical application of tranexamic acid together with Betadine solution in total knee arthroplasty. A randomised controlled study. orthop traumatol surg Res. 2015;101:83-87. [59] Hiippala ST, Strid LJ, Wennerstrand MI, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg 1997; 84: 839–44 [60] Andersson L, Nilsson IM, Colin S, Granstrand B, Melander B. Role of urokinase and tissue activator in sustained bleeding and the management thereof with EACA and AMCA. Ann N Y Acad Sci 1968; 146: 642–58 [61] Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol 1980; 33 (suppl. 14): 41–7. [62] Kaller H. Enterale Resorption, Verteilung und Elimination von 4- amino-methylcyclo-hexancarbonsau¨re (AMCHA) und e-aminocapronsau¨re (EACS) beim Menschen. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol 1967; 256: 160–8 [63] Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57(6): 1005e1032. [64] Vuylstcke A, Saravanan P, Gertard C, et al. The impact of administration of tranexamic acid in reducing the use of red blood cells and other blood products in cardiac surgery. BMC Anaesthesiol. 2006;30(6):9. [65] Pharmacia & Upjohn. Cyklokapron. ABPI Compendium of Data Sheets and Summaries of Product characteristics 1998- 1999. London: Datapharm Publications Ltd [66] Horrow JC, Van Riper DF, Strong MD, et al. The dose-response relationship of tranexamic acid. Anesthesiology 1995 Feb; 82: 383-92 [67] Gilad O, Merlob P, Stahl B, Klinger G. Outcome following tranexamic acid exposure during breastfeeding. Breastfeed Med. 2014 Oct;9(8):407-10. [68] Shiohara T (2009) Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol 9: 316-321. [69] Pretel Irazabal M, Marques Martin L, Aguado Gil L, Idoate Gastearena MA( 2013) Tranexamic acid-induced toxic epidermal necrolysis. Ann Pharmacother 47: e16. [70] Song Y, Izumi N, Potts LB, Yoshida A (2014) Tranexamic acid-induced ligneous conjunctivitis with renal failure showed reversible hypoplasminogenaemia. BMJ Case Rep 2014 [71] Samanta S, Jain K, Batra YK (2013) Global left and right ventricular dysfunction after tranexamic acid administration in a polytrauma patient. Ann Card Anaesth 16: 305-307 [72] R.S. Vardanyan, V.J. Hruby. Synthesis of Essential Drugs. Elsevier. 2006; 323-335 [73] Christopher J. Dunn and Karen L. Goa. Tranexamic Acid A Review of its Use in Surgery and Other Indications. Adis International Limited, Auckland, New Zealand. 1999 Jun; 57 (6): 1005-1032.
Copyright
Copyright © 2023 Mr. A. Prathap, Roja M K, Samyuktha D V, Saranya R, Shruthi D, Priyadarshini P. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET55659
Publish Date : 2023-09-07
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

