Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Understanding Pathways, Substrates, and Media Optimization to Maximize Microbial Biosurfactant Production
Authors: Nikhil Rawal, Rukhsar Ansari
DOI Link: https://doi.org/10.22214/ijraset.2024.60043
Certificate: View Certificate
Abstract
The production of amphiphilic chemicals, known as biosurfactants, by different microorganisms is essential for lowering interfacial and surface tension. The pathways of biosurfactant synthesis are examined in this review, with a focus on the influence of carbon substrates on biosynthesis. It also covers the variety of substrates used in the production of biosurfactants, such as lignocellulosic wastes, agro-industrial wastes, oil processing byproducts, starch-rich wastes, industrial wastes derived from plants and animals, fish and chicken wastes, fruit and vegetable wastes, and wastes from fish and poultry operations. In addition, the optimization of media for the formation of biosurfactants is discussed, with particular attention to temperature, pH, concentration of salt, and sources of carbon and nitrogen. It is crucial to comprehend these routes and optimization techniques in order to address the economic and environmental issues related to waste management and increase the efficiency of biosurfactant production.
Introduction
I. INTRODUCTION
Biosurfactants are amphiphilic substances that are generated extracellularly or on living surfaces, primarily microbial cell surfaces. They have hydrophobic and hydrophilic moieties that lower surface tension and interfacial tension between individual molecules at the interface, respectively [1]. Microbial surfactants are a significant class of compounds that are produced by filamentous fungus, yeasts, and bacteria. These are amphiphilic compounds that accumulate at immiscible interfaces to reduce the interfacial tension and surface tension. These chemicals are also formed during the stationary phase of microbial development. They are categorised as glycolipids, lipopeptides, phospholipids, and polymeric or particulate molecules based on their chemical makeup. Long-chain fatty acids, hydroxyl fatty acids, or a-alkyl-b-hydroxyl fatty acids are the typical components of the hydrophobic moiety. In the case of the hydrophilic section, an alcohol, phosphate, carbohydrate, amino acid, or cyclic peptide may be present [2].A diverse range of microorganisms synthesise biosurfactants, a heterogeneous category of secondary metabolites having surface-active characteristics such as Serratia rubidaea, Pseudomonas aeruginosa, Bacillus circulans, Bacillus subtilis, Starmerella bombicola, Aureobasidium thailandense, Candida lipolytica, Pleurotus djamoretc, Nocardiopsis lucentensis [3,4,5,6,7].
II. PATHWAYS OF BIOSURFACTANTS PRODUCTION
Studies indicate that biosurfactant can be synthesized through four distinct pathways, each dependent on the availability and nature of carbon sources in microbial fermentation. These pathways are elucidated as follows:
- Synthesis of both carbohydrates and lipids: In this pathway, precursor molecules for biosurfactant synthesis are derived from both carbohydrate and lipid sources. The microbial metabolism simultaneously activates pathways for carbohydrate and lipid synthesis, leading to the production of biosurfactants containing both components [8,9].
- Synthesis of carbohydrates with carbon source chain length-dependent lipid synthesis: Here, carbohydrates serve as the primary carbon source, while lipid synthesis depends on the length of the carbon substrate's chain present in the medium. Short-chain carbon substrates might favor lipid synthesis over carbohydrate synthesis, leading to the production of biosurfactants enriched in lipids [10,11].
- Synthesis of lipids with substrate-dependent carbohydrate synthesis: In this pathway, lipids are synthesized while carbohydrate synthesis is contingent upon the substrate utilized. The microbial metabolism primarily focuses on lipid synthesis, utilizing available substrates for lipid production while carbohydrate synthesis occurs depending on the specific substrate used [12].
- Synthesis of both carbohydrates and lipids dependent on substrate: This pathway involves the synthesis of both carbohydrates and lipids for biosurfactant production, with the synthesis of both components influenced by the characteristics of the substrate. The microbial metabolism adjusts its pathways to accommodate the available substrate, leading to the production of biosurfactants containing both carbohydrates and lipids [13, 10].
The choice of carbon source in microbial fermentation significantly impacts the production of biosurfactants by regulating precursor molecule availability and metabolic pathways. For instance, when simple carbohydrates are the primary carbon source, microbial metabolism prioritizes glycolysis to generate fatty acids, essential for lipid synthesis. Conversely, when hydrocarbons are utilized as carbon sources, microbial metabolism predominantly activates lipolytic routes and gluconeogenesis, facilitating the synthesis of fatty acids or sugars necessary for biosurfactant production [14,15,16].
In summary, the pathways of biosurfactant production are intricately linked to the metabolic processes of microbial organisms, which adapt their metabolic pathways according to the available carbon sources. This understanding underscores the importance of selecting appropriate carbon sources in microbial fermentation processes to optimize biosurfactant production [17,18,19].
III. SUBSTRATES INVOLVED IN BIOSURFACTANTS PRODUCTION
Many low-cost waste materials have been investigated as biosurfactant production substrates over the last ten years, resulting in an efficient cost-cutting approach together with the critical waste management. There is a huge potential for producing biosurfactants from a range of inexpensive, renewable industrial wastes. Food and agroindustry-related residues are prominent among them [20,21,22].
- Agro-industrial wastes: Based on studies, there is a significant increase in the production of generation of agro industrial byproducts. In order to improve the sustainability of these agro-industrial operations, it is imperative to either decrease wastage or employ waste products and/or effluents in processes that might produce other valuable goods, such surfactants [23].
- Oil processing waste & byproducts: Vegetable oil processing results in a significant amount of waste, primarily composed of fats, oils, and other related substances. Potent pollutants, these leftovers have the potential to pollute water and soil. The limited degradability of the lipid molecules they contain may be the reason for their ibility to function as pollutants. However, research has demonstrated that using soybean oil refinery waste and olive oil mill effluents as a substrate, microbial species such as pseudomonas can synthesise rhamnolipids. Similar investigations have shown that Candida sphaerica may effectively create biosurfactants from groundnut oil refinery waste. Other micro-bial strains, such as Bacillus subtilis, Starmerella bombicola, Trametes versicolor, etc., have also been effectively used to produce biosurfactants from oil industry wastes [24,25,26].
- Starch rich waste: A significant volume of wastewater that is high in starch and husks from the extraction of starch from rice, wheat, cassava, potatoes, and other crops is produced. This wastewater can be utilised as a feedstock to make a variety of goods, including bio-surfactants. Potato substrate was tested as an unorthodox carbon source for surfactant synthesis using Bacillus subtilis. It was also utilised to turn cassava wastewater into bio-surfactants. Additionally, lipopeptides were produced by Bacillus amyloliquefaciens using rice straw and soybean flour as substrates [27, 28, 29 ].
2. Industrial wastes from animal origin: Large amounts of animal fat and tallow are available from the meat processing industries, and these substances are utilised to cook food. Using the yeast C. bombicola, researchers produced sophorolipids biosurfactants using animal fat. Growth was inadequate when fat was the only carbon source available; however, the best degree of growth was obtained when 10% glucose and 10% fat were combined, suggesting that the medium needed to have an additional carbon source [30, 31 ].
- Fish waste: Around 60% of the weight of the fish is made up of fish wastes, which include fish bones, fish skin, fish head, red flesh, and viscera that are produced during the processing of fish. Due to their high concentration of suspended particles, organic carbon, and nitrogen, those wastes have the potential to pollute the environment and even create a number of health issues. Proteins, polyunsaturated fats, and minerals, among other substances with a high organic content, can be used as nutrition and then eliminated at the same time. Their widespread use as fishmeal for animal feed has resulted in significant environmental degradation and minimal economic returns. Fish peptones isolated from fish wastes have been shown in earlier research to have the capacity to promote the proliferation of microorganisms. Compared to other waste materials, fewer attempts have been made to date to produce biosurfactants from fish wastes. Therefore, it is imperative to do more thorough research on the manufacture of biosurfactants using fish wastes as an environmentally benign substitute to fully utilise these fish wastes [32, 33, 34, 35, 36, 37].
- Chicken waste: One significant environmental issue is keratinous waste from slaughterhouses, chicken processing, fur, and leather manufacturing. Proteolytic hydrolysis cannot break down keratin, which is the third most common polymer. Although the majority of research focuses on the production of keratinase enzymes, keratinolytic microorganisms can be utilised to create products with additional value. Studies indicate that by employing chicken tallow as a substrate, a new cationic biosurfactant was obtained from Alcaligenesaquatilis sp. The use of cationic biosurfactant was a novel strategy for the in situ bioremoval of Cr III from the soil. The effectiveness of removal was assessed, and the toxicity analysis was conducted on fenugreek, maize, and ridge gourd [38, 39, 40, 41, 42].
3. Industrial wastes from plant origin: P. aeruginosa (Strain GS3) was used by the researchers to produce rhamnolipid biosurfactant; the primary sources of carbon and nitrogen were molasses and corn-steep liquor. The combination of 7% (v/v) molasses and 0.5% (v/v) corn-steep liquor waste resulted in the highest biosurfactant production [69, 84]. Being a low-value byproduct of processing soybeans, soy molasses has a high potential sugar content that can be fermented, along with other growth factors that support microbial development, making it an affordable feedstock. According to research, soy molasses can be utilised in fermentation processes to create industrial compounds such poly-hydroxyalkanoates, lactic acid, butanol, sophorolipids, and biosurfactants. For the low-cost generation of sophorolipids biosurfactant by C. bombicola, researchers developed a soy molasses-based medium [43, 44, 45, 46].
- Fruit & vegetable wastes: Fruit peels and vegetable peels that are processed for their juices generate a large amount of garbage. Biosurfactant production can be facilitated by using waste materials such peels from apples, bananas, and oranges. According to studies, surface tension can be lowered by up to 17% by using cashew apple juice. One more waste product that could be used to produce biosurfactants is banana peels. Pseudomonas aeruginosa has been utilised to generate rhamnolipids from peels of carrot, lime, and orange varieties [47, 48, 49, 50, 51, 52].
- Lignocellulosic wastes: The potential of lignocellulose, a plentiful supply of organic carbon, in the synthesis of biosurfactants has been investigated. Using lignocellulosic substrates, strains of Lactobacillus pentosus and Bacillus tequilensis have been employed to generate biosurfactants. These substrates are reasonably priced and have a wide range of uses. However, pre-treatment procedures including drying, chemical/enzymatic hydrolysis, pre-hydrolysis, and particle size reduction drive up the cost of producing biosurfactants. Cutaneotrichosporon mucoides, Lactobacillus paracasei, Starmerella bombicola, and C. bombicola are among the microbe strains that consume lignocellulosic wastes [53, 54, 55, 56, 57 ]
IV. MEDIA OPTIMIZATION FOR BIOSURFACTANT PRODUCTION
Many investigations on media optimization, particularly for the most well known biosurfactant producers including Pseudomonas, Bacillus, and Candida species have been conducted within the past few years. The most widely researched factors for the generation of biosurfactants in shaking flasks and large-scale fermenters have been discovered to be the kind and quantity of carbon and nitrogen sources in the media, as well as the type and ratio of metal cations [58].
Meat extracts, yeast extracts, ammonium sulphate, ammonium nitrate, sodium nitrate, urea peptone, and malt extracts have all been used to make biosurfactants. Yeast extract is the most common source of nitrogen utilised in the synthesis of biosurfactants, but the amount used varies depending on the organism and growing medium. For the best surfactant synthesis, P. aeruginosa prefers nitrate, while Arthrobacter paraffineus prefers ammonium salts and urea as nitrogen sources [59, 60 ].
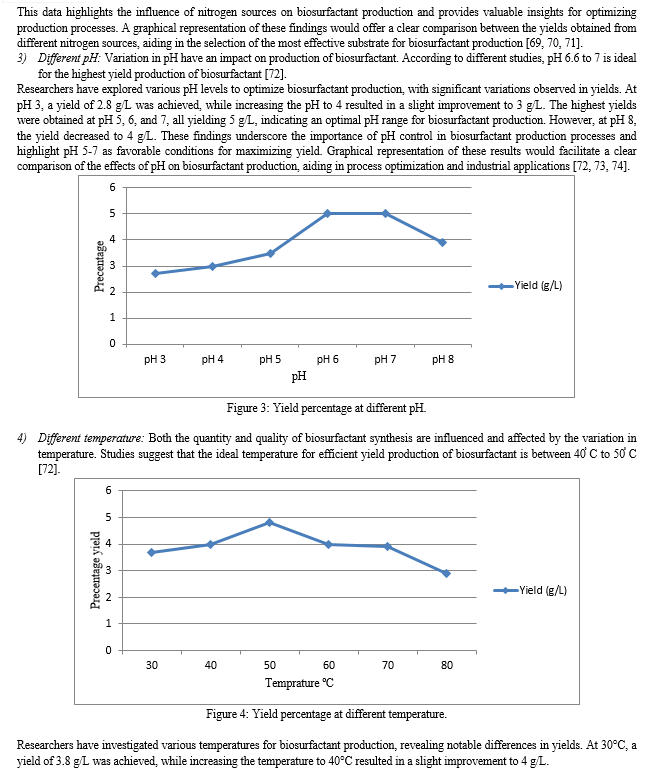
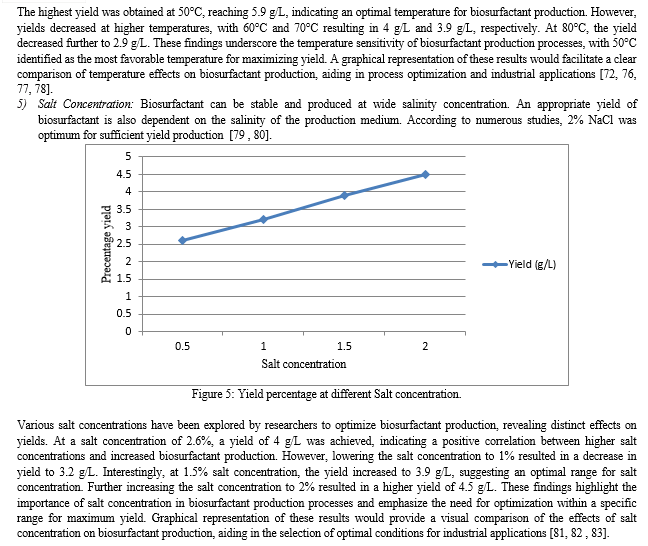
Since many factors can influence the growth and metabolism of microorganisms during fermentative synthesis, achieving the optimal biosurfactant yield can be challenging. The ideal combination of substrates for a certain culture medium has been the subject of numerous investigations aimed at facilitating intracellular diffusion and the production of desired compounds [61, 62, 63]. For optimal biosurfactant synthesis, the growing parameters of a selected strain of microbe must be specified. The amount of carbon and nitrogen available, the amount of lipophilic substrate, the availability of micronutrients, the size of the inoculum, temperature, pH, aeration, and agitation speed are all important variables. Even if most microorganisms that create biosurfactants do so under more restricted conditions, it's still necessary to investigate the growth phase that yields the best rate of production. Statistical methods can be used to optimise the chemical and physical parameters of the fermentation process. This allows one to study the effects of interactions between the various variables and determine the optimal culture conditions for the highest production of biosurfactants at the lowest possible cost [15, 56, 85, 94]. The parameters affecting media optimization in production are as follows:
1) Carbon Sources: The type of carbon sources affects & influences the synthesis of biosurfactants in terms of both quantity & quality. Studies indicate that diseal, crude oil, fructose, glucose, and sucrose are excellent sources of carbon substrate for the synthesis of biosurfactants [64].

Conclusion
In conclusion, the production of biosurfactants offers a promising avenue for sustainable surfactant solutions, leveraging renewable resources and diverse microorganisms. Through the examination of biosurfactant synthesis pathways and the influence of carbon substrates, it becomes evident that microbial metabolism adapts to available carbon sources, impacting biosurfactant yields and characteristics. Furthermore, the utilization of various substrates for biosurfactant production, including agro-industrial wastes, oil processing byproducts, starch-rich wastes, industrial wastes from both plant and animal origins, fish and chicken wastes, fruit and vegetable wastes, and lignocellulosic wastes, highlights the potential for waste valorization and efficient waste management practices. Optimization of media parameters such as temperature, pH, salt concentration, and carbon and nitrogen sources is crucial for enhancing biosurfactant production efficiency. Understanding the interplay between these factors enables researchers to tailor fermentation processes for optimal biosurfactant yields. While challenges such as production costs, scalability, and consistency persist, ongoing research and technological advancements hold promise for addressing these issues and meeting the increasing demand for eco-friendly surfactant solutions. By continuing to explore diverse microbial strains and substrate sources, as well as refining media optimization techniques, the field of biosurfactant production can contribute significantly to sustainable waste management practices and environmental preservation efforts.
References
[1] Qureshi, N., Lolas, A., & Blaschek, H. P. (2001). Soy molasses as fermentation substrate for production of butanol using Clostridium beijerinckii BA101. Journal of industrial Microbiology and Biotechnology, 26(5), 290-295. [2] Rocha, M. V. P., Oliveira, A. H. S., Souza, M. C. M., & Gonçalves, L. R. B. (2006). Natural cashew apple juice as fermentation medium for biosurfactant production by Acinetobacter calcoaceticus. World Journal of Microbiology and Biotechnology, 22, 1295-1299 [3] Bezerra, K. G., Gomes, U. V., Silva, R. O., Sarubbo, L. A., & Ribeiro, E. (2019). The potential application of biosurfactant produced by Pseudomonas aeruginosa TGC01 using crude glycerol on the enzymatic hydrolysis of lignocellulosic material. Biodegradation, 30, 351-361. [4] Boopathy, R., Bonvillain, C., Fontenot, Q., & Kilgen, M. (2007). Biological treatment of low-salinity shrimp aquaculture wastewater using sequencing batch reactor. International biodeterioration & biodegradation, 59(1), 16-19. [5] Gaur, V. K., Sharma, P., Gupta, S., Varjani, S., Srivastava, J. K., Wong, J. W. C., et al. (2022). Opportunities and challenges in omics approaches for biosurfactant production and feasibility of site remediation: strategies and advancements. Environ. Technol. Innov.25:102132. doi: 10.1016/J.ETI.2021.102132 [6] Konishi, M., Yoshida, Y., & Horiuchi, J. I. (2015). Efficient production of sophorolipids by Starmerella bombicola using a corncob hydrolysate medium. Journal of bioscience and bioengineering, 119(3), 317-322. [7] Waghmode, S., Kulkarni, C., Shukla, S., Sursawant, P., & Velhal, C. (2014). Low cost production of biosurfactant from different substrates and their comparative study with commercially available chemical surfactant. Int J Sci Technol Res, 3, 146-149. [8] Chikezie Ogbu, C., & Nnaemeka Okey, S. (2023). Agro-Industrial Waste Management: The Circular and Bioeconomic Perspective. IntechOpen. doi: 10.5772/intechopen.109181. [9] Sharma, P., Gaur, V. K., Kim, S. H., & Pandey, A. (2020). Microbial strategies for bio-transforming food waste into resources. Bioresource technology, 299, 122580. [10] El-Sheshtawy, H. S., Aiad, I., Osman, M. E., Abo-ELnasr, A. A., and Kobisy, A. S. (2015). Production of biosurfactant from Bacillus licheniformis for microbial enhanced oil recovery and inhibition the growth of sulfate reducing bacteria. Egypt. J. Pet. 24, 155–162. doi: 10.1016/J.EJPE.2015.05.005 [11] Rodríguez, D. M., de Souza Mendonça, R., de Souza, A. F., da Silva Ferreira, I. N., da Silva Andrade, R. F., & Campos-Takaki, G. M. (2022). Solid-state fermentation for low-cost production of biosurfactant by promising Mucor hiemalis UCP 1309. Research, Society and Development, 11(6), e25211628817-e25211628817. [12] Drakontis, C. E., & Amin, S. (2020). Biosurfactants: Formulations, properties, and applications. Current Opinion in Colloid & Interface Science, 48, 77-90. [13] Domínguez Rivera, Á., Martínez Urbina, M. Á., & López y López, V. E. (2019). Advances on research in the use of agro-industrial waste in biosurfactant production. World J [14] Rane, A. N., Baikar, V. V., Ravi Kumar, V., & Deopurkar, R. L. (2017). Agro-industrial wastes for production of biosurfactant by Bacillus subtilis ANR 88 and its application in synthesis of silver and gold nanoparticles. Frontiers in Microbiology, 8, 244827. [15] Rocha, M. V. P., Oliveira, A. H. S., Souza, M. C. M., & Gonçalves, L. R. B. (2006). Natural cashew apple juice as fermentation medium for biosurfactant production by Acinetobacter calcoaceticus. World Journal of Microbiology and Biotechnology, 22, 1295-1299 [16] Safari, R., Motamedzadegan, A., Ovissipour, M., Regenstein, J. M., Gildberg, A., & Rasco, B. (2012). Use of hydrolysates from yellowfin tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food and Bioprocess Technology, 5, 73-79 [17] Sarubbo, L. A., Maria da Gloria, C. S., Durval, I. J. B., Bezerra, K. G. O., Ribeiro, B. G., Silva, I. A., ... & Banat, I. M. (2022). Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochemical Engineering Journal, 181, 108377. [18] Sharma, P., Gaur, V. K., Kim, S. H., & Pandey, A. (2020). Microbial strategies for bio-transforming food waste into resources. Bioresource technology, 299, 122580. [19] Velioglu, Z., & Ozturk Urek, R. (2014). Concurrent biosurfactant and ligninolytic enzyme production by Pleurotus spp. in solid-state fermentation. Applied biochemistry and biotechnology, 174, 1354-1364. [20] Begum, W., Saha, B., & Mandal, U. (2023). A comprehensive review on production of bio-surfactants by bio-degradation of waste carbohydrate feedstocks: an approach towards sustainable development. RSC advances, 13(36), 25599-25615. [21] Santos, D. K. F., Meira, H. M., Rufino, R. D., Luna, J. M., & Sarubbo, L. A. (2017). Biosurfactant production from Candida lipolytica in bioreactor and evaluation of its toxicity for application as a bioremediation agent. Process Biochemistry, 54, 20-27. [22] Solaiman, D. K., Ashby, R. D., Hotchkiss, A. T., & Foglia, T. A. (2006). Biosynthesis of medium-chain-length poly (hydroxyalkanoates) from soy molasses. Biotechnology letters, 28, 157-162. [23] Sharma, P., Gaur, V. K., Kim, S. H., & Pandey, A. (2020). Microbial strategies for bio-transforming food waste into resources. Bioresource technology, 299, 122580. [24] Jahan, R., Bodratti, A. M., Tsianou, M., and Alexandridis, P. (2020). Biosurfactants, natural alternatives to synthetic surfactants: physicochemical properties and applications. Adv. Colloid Interf. Sci. 275:102061. doi: 10.1016 J.CIS.2019.102061 [25] Kosaric, N. (1992). Biosurfactants in industry, Pure & Appl. Chern, 64(11), 1731-1737. [26] Onbasli, D., & Aslim, B. (2009). Biosurfactant production in sugar beet molasses by some Pseudomonas spp. J Environ Biol, 30(1), 161-163. [27] Bjerk, T. R., Severino, P., Jain, S., Marques, C., Silva, A. M., Pashirova, T., & Souto, E. B. (2021). Biosurfactants: properties and applications in drug delivery, [28] Habib, S., Ahmad, S. A., Johari, W. L. W., Shukor, M. Y. A., Alias, S. A., Smykla, J., et al. (2020). Production of Lipopeptide biosurfactant by a hydrocarbon-degrading Antarctic Rhodococcus. Int. J. Mol. Sci. 21:6138. doi: 10.3390/IJMS21176138 [29] Onbasli, D., & Aslim, B. (2009). Biosurfactant production in sugar beet molasses by some Pseudomonas spp. J Environ Biol, 30(1), 161-163. [30] Domínguez Rivera, Á., Martínez Urbina, M. Á., & López y López, V. E. (2019). Advances on research in the use of agro-industrial waste in biosurfactant production. World J [31] Santos, D. K. F., Meira, H. M., Rufino, R. D., Luna, J. M., & Sarubbo, L. A. (2017). Biosurfactant production from Candida lipolytica in bioreactor and evaluation of its toxicity for application as a bioremediation agent. Process Biochemistry, 54, 20-27. [32] Brasileiro, P. P., de Almeidab, D. G., de Lunaa, J. M., Rufinoa, R. D., dos Santosa, V. A., & Sarubboa, L. A. (2015). Optimization of biosurfactant production from Candida guiliermondii using a Rotate Central Composed Design. CHEMICAL ENGINEERING, 43. [33] Joy, S., Rahman, P. K., Khare, S. K., & Sharma, S. (2019). Production and characterization of glycolipid biosurfactant from Achromobacter sp.(PS1) isolate using one-factor-at-a-time (OFAT) approach with feasible utilization of ammonia-soaked lignocellulosic pretreated residues. Bioprocess and biosystems engineering, 42, 1301-1315. [34] Jiménez-Peñalver, P., Rodríguez, A., Daverey, A., Font, X., and Gea, T. (2019). Use of wastes for sophorolipids production as a transition to circular economy: state of the art and perspectives. Rev. Environ. Sci 18, 413–435. doi: 10.1007/S11157-019-09502-3 [35] Saharan, B. S., Sahu, R. K., & Sharma, D. (2011). A review on biosurfactants: fermentation, current developments and perspectives. Genetic Engineering and Biotechnology Journal, 2011(1), 1-14. [36] Velioglu, Z., & Ozturk Urek, R. (2014). Concurrent biosurfactant and ligninolytic enzyme production by Pleurotus spp. in solid-state fermentation. Applied biochemistry and biotechnology, 174, 1354-1364. [37] Zinjarde, S. S., & Pant, A. (2002). Emulsifier from a tropical marine yeast, Yarrowia lipolytica NCIM 3589. Journal of Basic Microbiology: An International Journal on Biochemistry, Physiology,Genetics, Morphology, and Ecology of Microorganisms, 42(1), 67-73. [38] Gurjar, J., & Sengupta, B. (2015). Production of surfactin from rice mill polishing residue by submerged fermentation using Bacillus subtilis MTCC 2423. Bioresource technology, 189, 243-249. [39] Luna JM, Rufno RD, Jara AMAT, Brasileiro PPF, Sarubbo LA. Environmental applications of the biosurfactant produced by Candida sphaerica cultivated in low-cost substrates. Colloids Surf A Physicochem Eng Asp. 2015;480:413–8. [40] Martinez-Burgos, W. J., Sydney, E. B., Medeiros, A. B. P., Magalhães, A. I., de Carvalho, J. C., Karp, S. G., ... & Soccol, C. R. (2021). Agro-industrial wastewater in a circular economy: Characteristics, impacts and applications for bioenergy and biochemicals. Bioresource technology, 341, 125795. [41] Phulpoto, I. A., Yu, Z., Hu, B., Wang, Y., Ndayisenga, F., Li, J., et al. (2020). Production and characterization of surfactin-like biosurfactant produced by novel strain Bacillus nealsonii S2MT and it’s potential for oil contaminated soil remediation. Microb. Cell [42] Takahashi, M., Morita, T., Wada, K., Hirose, N., Fukuoka, T., Imura, T., & Kitamoto, D. (2011). Production of sophorolipid glycolipid biosurfactants from sugarcane molasses using Starmerella bombicola NBRC 10243. Journal of Oleo Science, 60(5), 267-273 [43] Rahman, K. S., Rahman, T. J., Kourkoutas, Y., Petsas, I., Marchant, R., & Banat, I. M. (2003). Enhanced bioremediation of n-alkane in petroleum sludge using bacterial consortium amended with rhamnolipid and micronutrients. Bioresource technology, 90(2), 159-168. [44] Santos, D. K. F., Meira, H. M., Rufino, R. D., Luna, J. M., & Sarubbo, L. A. (2017). Biosurfactant production from Candida lipolytica in bioreactor and evaluation of its toxicity for application as a bioremediation agent. Process Biochemistry, 54, 20-27. [45] Solaiman, D. K., Ashby, R. D., Nuñez, A., & Foglia, T. A. (2004). Production of sophorolipids by Candida bombicola grown on soy molasses as substrate. Biotechnology letters, 26, 1241-1245. [46] Sundaram, T., Govindarajan, R. K., Vinayagam, S., Krishnan, V., Nagarajan, S., Gnanasekaran, G. R., ... & Rajamani Sekar, S. K. (2024). Advancements in biosurfactant production using agro-industrial waste for industrial and environmental applications. Frontiers in Microbiology, 15, 1357302. [47] Chikezie Ogbu, C., & Nnaemeka Okey, S. (2023). Agro-Industrial Waste Management: The Circular and Bioeconomic Perspective. IntechOpen. doi: 10.5772/intechopen.109181. [48] Ciurko, D., Czy?nikowska, ?., Kancelista, A., ?aba, W., and Janek, T. (2022).Sustainable production of biosurfactant from agro-industrial oil wastes by Bacillus subtilis and its potential application as antioxidant and ACE inhibitor. Int. J. Mol. Sci.23:10824. doi: 10.3390/IJMS231810824 [49] Kumar, P. S., Mohanakrishna, G., Hemavathy, R. V., Rangasamy, G., & Aminabhavi, T. M. (2023). Sustainable production of biosurfactants via valorisation of industrial wastes as alternate feedstocks. Chemosphere, 312, 137326. [50] Onbasli, D., & Aslim, B. (2009). Biosurfactant production in sugar beet molasses by some Pseudomonas spp. J Environ Biol, 30(1), 161-163. [51] Roy, A. R. P. I. T. A. (2014). Production and characterization of biosurfactant from bacterial isolates. Netaji SubhasInstitue of Technology Azad, Dwarka, New Delhi [52] Solaiman, D. K., Ashby, R. D., Hotchkiss, A. T., & Foglia, T. A. (2006). Biosynthesis of medium-chain-length poly (hydroxyalkanoates) from soy molasses. Biotechnology letters, 28, 157-162. [53] Kiran, G. S., Thomas, T. A., & Selvin, J. (2010). Production of a new glycolipid biosurfactant from marine Nocardiopsis lucentensis MSA04 in solid-state cultivation. Colloids and Surfaces B: Biointerfaces, 78(1), 8-16. [54] Bhange, K., Chaturvedi, V., & Bhatt, R. (2016). Simultaneous production of detergent stable keratinolytic protease, amylase and biosurfactant by Bacillus subtilis PF1 using agro industrial waste. Biotechnology reports, 10, 94-104. [55] Konkol, D., Szmigiel, I., Dom?a?-K?dzia, M., Ku?a?y?ski, M., Krasowska, A., Opali?ski, S., ... & ?ukaszewicz, M. (2019). Biotransformation of rapeseed meal leading to production of polymers, biosurfactants, and fodder. Bioorganic chemistry, 93, 102865 [56] Patel, R. M., & Desai, A. J. (1997). Biosurfactant production by Pseudomonas aeruginosaGS3 from molasses. Letters in Applied Microbiology, 25(2), 91-94. [57] Onbasli, D., & Aslim, B. (2009). Biosurfactant production in sugar beet molasses by some Pseudomonas spp. J Environ Biol, 30(1), 161-163. [58] Solaiman, D. K., Ashby, R. D., Nuñez, A., & Foglia, T. A. (2004). Production of sophorolipids by Candida bombicola grown on soy molasses as substrate. Biotechnology letters, 26, 1241-1245. [59] Adamczak, M., & Bednarski, W. O. (2000). Influence of medium composition and aeration on the synthesis of biosurfactants produced by Candida antarctica. Biotechnology Letters, 22(4), 313-316. [60] Bezerra, K. G., Gomes, U. V., Silva, R. O., Sarubbo, L. A., & Ribeiro, E. (2019). The potential application of biosurfactant produced by Pseudomonas aeruginosa TGC01 using crude glycerol on the enzymatic hydrolysis of lignocellulosic material. Biodegradation, 30, 351-361. [61] Chebbi, A., Franzetti, A., Duarte Castro, F., Gomez Tovar, F. H., Tazzari, M., Sbaffoni, S., & Vaccari, M. (2021). Potentials of winery and olive oil residues for the production of rhamnolipids and other biosurfactants: a step towards achieving a circular economy model. Waste and Biomass valorization, 12, 4733-4743. [62] Kumar, A. P., Janardhan, A., Viswanath, B., Monika, K., Jung, J. Y., & Narasimha, G. (2016). Evaluation of orange peel for biosurfactant production by Bacillus licheniformis and their ability to degrade naphthalene and crude oil. 3 Biotech, 6, 1-10. [63] Sarubbo, L. A., Maria da Gloria, C. S., Durval, I. J. B., Bezerra, K. G. O., Ribeiro, B. G., Silva, I. A., ... & Banat, I. M. (2022). Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochemical Engineering Journal, 181, 108377. [64] Velioglu, Z., & Ozturk Urek, R. (2014). Concurrent biosurfactant and ligninolytic enzyme production by Pleurotus spp. in solid-state fermentation. Applied biochemistry and biotechnology, 174, 1354-1364. [65] El-Sheshtawy, H. S., Aiad, I., Osman, M. E., Abo-ELnasr, A. A., and Kobisy, A. S. (2015). Production of biosurfactant from Bacillus licheniformis for microbial enhanced oil recovery and inhibition the growth of sulfate reducing bacteria. Egypt. J. Pet. 24, 155–162. doi: 10.1016/J.EJPE.2015.05.005 [66] Sharma, P., Gaur, V. K., Kim, S. H., & Pandey, A. (2020). Microbial strategies for bio-transforming food waste into resources. Bioresource technology, 299, 122580. [67] Saharan, B. S., Sahu, R. K., & Sharma, D. (2011). A review on biosurfactants: fermentation, current developments and perspectives. Genetic Engineering and Biotechnology Journal, 2011(1), 1-14. [68] Ribeaux, D. R., Jackes, C. V., Takaki, G. M. C., Medeiros, A. D. M., Marinho, J., Lins, U., ... & Barreto, G. (2020). Innovative Production of Biosurfactant by Candida Tropicalis UCP 1613 through Solid-State Fermentation. CHEMICAL ENGINEERING, 79, 361-366. [69] Zouari, R., Ellouze-Chaabouni, S., & Ghribi-Aydi, D. (2014). Optimization of Bacillus subtilis SPB1 biosurfactant production under solid-state fermentation using by-products of a traditional olive mill factory. Achievements in the Life Sciences, 8(2), 162-169. [70] Adetunji, A. I., & Olaniran, A. O. (2021). Production and potential biotechnological applications of microbial surfactants: An overview. Saudi Journal of Biological Sciences, 28(1), 669-679. [71] Ahmad, Zulfiqar, et al. \"Estimation of biosurfactant yield produced by Klebseilla sp. FKOD36 bacteria using artificial neural network approach.\" Measurement 81 (2016): 163-173. [72] Rodríguez, D. M., de Souza Mendonça, R., de Souza, A. F., da Silva Ferreira, I. N., da Silva Andrade, R. F., & Campos-Takaki, G. M. (2022). Solid-state fermentation for low-cost production of biosurfactant by promising Mucor hiemalis UCP 1309. Research, Society and Development, 11(6), e25211628817-e25211628817. [73] Safari, R., Motamedzadegan, A., Ovissipour, M., Regenstein, J. M., Gildberg, A., & Rasco, B. (2012). Use of hydrolysates from yellowfin tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food and Bioprocess Technology, 5, 73-79. [74] Panjiar, N., Mattam, A. J., Jose, S., Gandham, S., & Velankar, H. R. (2020). Valorization of xylose-rich hydrolysate from rice straw, an agroresidue, through biosurfactant production by the soil bacterium Serratia nematodiphila. Science of the Total Environment, 729, 138933. [75] Martinez-Burgos, W. J., Sydney, E. B., Medeiros, A. B. P., Magalhães, A. I., de Carvalho, J. C., Karp, S. G., ... & Soccol, C. R. (2021). Agro-industrial wastewater in a circular economy: Characteristics, impacts and applications for bioenergy and biochemicals. Bioresource technology, 341, 125795. [76] Nalini, S., & Parthasarathi, R. (2018). Optimization of rhamnolipid biosurfactant production from Serratia rubidaea SNAU02 under solid-state fermentation and its biocontrol efficacy against Fusarium wilt of eggplant. Annals of Agrarian Science, 16(2), 108-115. [77] Jahan, R., Bodratti, A. M., Tsianou, M., and Alexandridis, P. (2020). Biosurfactants, natural alternatives to synthetic surfactants: physicochemical properties and applications. Adv. Colloid Interf. Sci. 275:102061. doi: 10.1016 J.CIS.2019.102061 [78] Jiménez-Peñalver, P., Rodríguez, A., Daverey, A., Font, X., and Gea, T. (2019). Use of wastes for sophorolipids production as a transition to circular economy: state of the art and perspectives. Rev. Environ. Sci 18, 413–435. doi: 10.1007/S11157-019-09502-3 [79] Jimoh, A. A., Senbadejo, T. Y., Adeleke, R., and Lin, J. (2021). Development and genetic engineering of hyper-producing microbial strains for improved synthesis of biosurfactants. Mol. Biotechnol. 634, 267–288. doi: 10.1007/S12033-021-00302-1 [80] Invally, K., Sancheti, A., and Ju, L. K. (2019). A new approach for downstream purification of rhamnolipid biosurfactants. Food Bioprod. Process. 114, 122–131. doi: 10.1016/J.FBP.2018.12.00 [81] Jadhav, J. V., Pratap, A. P., & Kale, S. B. (2019). Evaluation of sunflower oil refinery waste as feedstock for production of sophorolipid. Process Biochemistry, 78, 15-24. [82] Panjiar, N., Mattam, A. J., Jose, S., Gandham, S., & Velankar, H. R. (2020). Valorization of xylose-rich hydrolysate from rice straw, an agroresidue, through biosurfactant production by the soil bacterium Serratia nematodiphila. Science of the Total Environment, 729, 138933. [83] Onbasli, D., & Aslim, B. (2009). Biosurfactant production in sugar beet molasses by some Pseudomonas spp. J Environ Biol, 30(1), 161-163. [84] Saharan, B. S., Sahu, R. K., & Sharma, D. (2011). A review on biosurfactants: fermentation, current developments and perspectives. Genetic Engineering and Biotechnology Journal, 2011(1), 1-14. [85] Twigg, M. S., Baccile, N., Banat, I. M., Déziel, E., Marchant, R., Roelants, S., & Van Bogaert, I. N. (2021). Microbial biosurfactant research: time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microbial Biotechnology, 14(1), 147-170. [86] Johnson, P., Trybala, A., Starov, V., and Pinfield, V. J. (2021). Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interf. Sci. 288:102340. doi: 10.1016/J.CIS.2020.102340 [87] Youssef, N. H., Duncan, K. E., Nagle, D. P., Savage, K. N., Knapp, R. M., & McInerney, M. J. (2004). Comparison of methods to detect biosurfactant production by diverse microorganisms. Journal of microbiological methods, 56(3), 339-347. [88] Sharma, P., Gaur, V. K., Kim, S. H., & Pandey, A. (2020). Microbial strategies for bio-transforming food waste into resources. Bioresource technology, 299, 122580. [89] Waghmode, S., Kulkarni, C., Shukla, S., Sursawant, P., & Velhal, C. (2014). Low cost production of biosurfactant from different substrates and their comparative study with commercially available chemical surfactant. Int J Sci Technol Res, 3, 146-149. [90] Zhu, Z., Zhang, B., Cai, Q., Ling, J., Lee, K., & Chen, B. (2020). Fish waste based lipopeptide production and the potential application as a bio-dispersant for oil spill control. Frontiers in bioengineering and biotechnology, 8, 734. [91] Phulpoto, I. A., Yu, Z., Hu, B., Wang, Y., Ndayisenga, F., Li, J., et al. (2020). Production and characterization of surfactin-like biosurfactant produced by novel strain Bacillus nealsonii S2MT and it’s potential for oil contaminated soil remediation. Microb. Cell Factories 19, 1–12. doi: 10.1186/S12934-020-01402-4/FIGURES/5 [92] PENDYALA, S., & KULKARNI, M. STATISTICAL EVALUATION OF CHICKEN FEATHER WASTES AS SUBSTRATE FOR BIOSURFACTANT PRODUCTION BY BACILLUS VALLISMORTIS RMS25 [93] Martinez-Burgos, W. J., Sydney, E. B., Medeiros, A. B. P., Magalhães, A. I., de Carvalho, J. C., Karp, S. G., ... & Soccol, C. R. (2021). Agro-industrial wastewater in a circular economy: Characteristics, impacts and applications for bioenergy and biochemicals. Bioresource technology, 341, 125795. [94] L. Lange, Y.H. Huang, P.K. Busk, Microbial decomposition of keratin in nature-a new hypothesis of industrial relevance, Appl. Microbiol. Biotechnol. 100 (2016) 2083-2096 [95] Martins, V. G., Kalil, S. J., & Costa, J. A. V. (2009). In situ bioremediation using biosurfactant produced by solid state fermentation. World journal of microbiology and biotechnology, 25, 843-851 [96] Kourmentza, C., Freitas, F., Alves, V., & Reis, M. A. (2017). Microbial conversion of waste and surplus materials into high-value added products: the case of biosurfactants. Microbial Applications Vol. 1: Bioremediation and Bioenergy, 29-77. [97] Helvia, W. C. A., Rosileide, F. S. A., Dayana, M.-R., Vanessa, P. S., Patricia, C. V. S. M., Carlos, F. B. C. F., et al. (2017). Biochemical and molecular identification of newly isolated pigmented bacterium and improved production of biosurfactant. Afr. J. Microbiol. Res. 11, 945–954. doi: 10.5897/ajmr2016.8340 [98] Konishi, M., Yoshida, Y., & Horiuchi, J. I. (2015). Efficient production of sophorolipids by Starmerella bombicola using a corncob hydrolysate medium. Journal of bioscience and bioengineering, 119(3), 317-322. [99] L. Lange, Y.H. Huang, P.K. Busk, Microbial decomposition of keratin in nature-a new hypothesis of industrial relevance, Appl. Microbiol. Biotechnol. 100 (2016) 2083-2096 [100] Hollenbach, R., Bindereif, B., Van der Schaaf, U. S., Ochsenreither, K., & Syldatk, C. (2020). Optimization of glycolipid synthesis in hydrophilic deep eutectic solvents. Frontiers in Bioengineering and Biotechnology, 8, 382. [101] da Silva, A. F., Banat, I. M., Giachini, A. J., and Robl, D. (2021). Fungal biosurfactants, from nature to biotechnological product: bioprospection, production and potential applications. Bioprocess Biosyst. Eng. 4410, 2003–2034. doi: 10.1007/S00449-021-02597-5
Copyright
Copyright © 2024 Nikhil Rawal, Rukhsar Ansari. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET60043
Publish Date : 2024-04-09
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

