Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Tiny But Mighty: Unveiling the Nutritional Powerhouse of Watermelon Seeds
Authors: Ms. Aarti Labhane, Laiba Momin, Raj Patil, Ms. Reema Rani, Dr. Rupali Tasgaonkar, Sonal Nuche, Omkar Mohite
DOI Link: https://doi.org/10.22214/ijraset.2024.59308
Certificate: View Certificate
Abstract
The Curcubitaceae family includes the significant horticultural crop known as watermelon (Citrulus lantus). Many researchers have demonstrated watermelon\'s nutraceutical potential, making it a superior option for a functional diet. Numerous illnesses, including those connected to age, obesity, diabetes, ulcers, and several kinds of cancer, have been treated with watermelon. Citruline, lycopene, and other polyphenolic substances are among the significant phytochemicals of pharmacological significance that provide watermelon its therapeutic qualities. Watermelon serves as a crucial supplier of l-citrulline, a neutral-alpha amino acid that serves as a precursor to l-arginine, an essential amino acid required for the synthesis of proteins. Numerous health benefits were demonstrated in both in vitro and in vivo trials when l-citrulline and lycopene supplements were given. In the same way, the dietary similarly, watermelon consumption has been shown to be beneficial as a functional meal for weight management in people. In addition to the fruits, the seeds, sprouts, and leaves also showed therapeutic qualities in the extracts that were made from them. The benefits of watermelon for treating a variety of illnesses are covered in detail in this review.
Introduction
I. INTRODUCTION
Eating a diet rich in fruits and vegetables has several health advantages. A plant-based diet is considered healthier due to the abundance of phytochemicals, including flavonoids, lycopene’s, anthocyanins, phenols, and carotenoids, as well as vitamins and minerals. It reduces the chances of a number of awful illnesses, including cancer, heart disease, neurological conditions, and illnesses related to aging.
Plants yield a variety of products in the form of fruits and vegetables that are rich in nutraceutical potential, since they are a vast source of secondary metabolites that have pharmacological value. Similarly, watermelon consumption has been shown to be beneficial as a functional meal for weight management in people. In addition to the fruits, the seeds, sprouts, and leaves also showed therapeutic qualities in the extracts that were made from them. The benefits of watermelon for treating a variety of illnesses are covered in detail in this review.
A prominent member of the Curcubitaceae family of horticultural crops, watermelon is grown extensively for its mouthwatering fruits. Approximately 81% of the watermelon produced worldwide is grown in Asian nations. [1]
Watermelon serves as an essential source of rich phytochemicals that have both great nutritional value and potential medicinal applications. Watermelon, in instance, has high levels of lycopene, vitamin A, vitamin C, and antioxidant potential, making it an ideal functional food. [2, 3]
The presence of bioactive chemicals in watermelon has been linked to several health benefits, including a lower risk of cardiovascular disease, aging-related illnesses, obesity, diabetes, and a number of cancer-preventing properties. [4, 5, 6, 7].
Citrulline is a non-essential amino acid that Wada [8] discovered and isolated from watermelon in 1930. It is used in the production of arginine. Nitric oxide, an essential signaling molecule involved in a variety of neurological and immunological responses in both humans and animals, is synthesized endogenously by humans and requires the amino acid arginine. [9]
The watermelon seed's high arginine concentration which contributes to its therapeutic properties. [10]
This study discusses watermelon's several nutraceutical potentials and highlights its significance as an antioxidant, anti-inflammatory, cardiovascular protectant, and anti-cancerous food.
II. THERAPEUTIC EFFECTS
A. Cardiovascular Protection By Watermelon
Globally, cardiovascular disorders are the primary cause of the rising death rate. Furthermore, treating cardiovascular disease is expensive. Consequently, adopting a cardio-friendly diet and lifestyle would lower the disease's risk factors. Vegetables and fruits can counteract the harmful effects of cardiovascular illnesses. L-arginine and L-citrulline have the ability to reduce oxidative stress and inflammation.[11,12].On the other hand, consuming l-arginine and l-citrulline directly may cause gastrointestinal distress like nausea and diarrhea[13,14].Thus, consuming fruits high in l-citrulline—a precursor to l-arginine, an amino acid required for protein synthesis—like watermelon is essential for receiving the nutrients your body needs. Rats on high fat diets showed improvements in their lipid profiles, antioxidant status, and anti-inflammatory capabilities when given whole watermelon in powder form as a supplement [15]. Additionally, eating watermelon affected the expression of genes related to fat metabolism [15]. More specifically, the addition of watermelon and l-arginine improved the control of endothelial nitric oxide synthase gene expression in the liver. A ubiquitous signaling molecule that is essential for blood vessel relaxation, nitric oxide (NO) also lowers atherosclerosis by affecting lipid metabolism [15,16,17].On the contrary, in rats, watermelon supplementation down-regulated genes involved in lipid metabolism, including nuclear factor-kB (NF-κB), cyclooxygenase-2 (COX2), fatty acid synthase (FAS), 3-hydroxy-3methyl glutaryl-coA reductase (HMGCR), and sterol regulatory element binding protein (SERB) 1 and 2.[15]
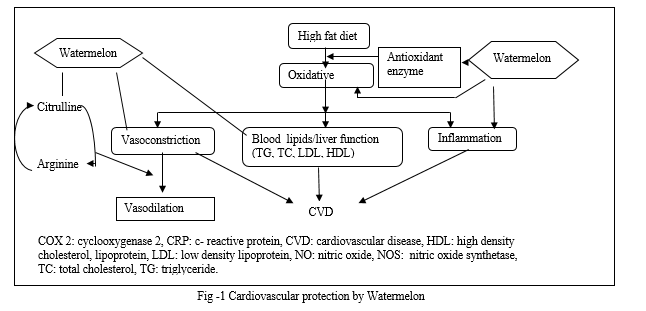
Of the aforementioned enzymes, FAS is crucial for the denovo synthesis of fatty acids, while HMGCR limits the rate at which cholesterol is synthesized [18]. In a similar vein, SREBP-1 and SREBP-2 control the transcription of genes related to the synthesis of cholesterol and fatty acids, respectively [19].The main causes of atherosclerosis are inflammation and oxidative stress. Serum levels of C-reactive protein are used as markers for systemic inflammation, which causes heart failure [20, 21]. Rats fed a high-fat diet had significantly lower blood levels of C-reactive protein after consuming watermelon [22]. Additionally, the expression of the Cox-2 enzyme, which is involved in the creation of prostaglandins that promote inflammation, was down regulated by watermelon. Additionally, Hong et al. [22] shown that the mechanism by which watermelon supplementation decreases the activity of Cox-2 and lowers the inflammatory response is comparable to that of non-steroidal anti-inflammatory medicines. Watermelon can lower cardiovascular disease risk factors in humans, according to a recent study [23, 24].Connolly et al. [24] reported that consuming watermelon on a daily basis for four weeks led to significant decreases in blood pressure, waist-to-hip ratio, body weight, and body mass index. Furthermore, the study states that supplementing with watermelon reduced the levels of triglycerides, low-density lipoprotein cholesterol, and reactive chemical called thiobarbituric acid, and enhanced antioxidant capacity in individuals who are obese [24]. Overall, it is clear that eating watermelon on a regular basis lowers the risk factors related to long-term conditions like cardiovascular disease.
B. Obesity
Obesity is a worrisome global public health concern that is associated with important metabolic illnesses such as diabetes and diseases related to lifestyle choices. Obesity is mostly caused by an unhealthy lifestyle and bad eating habits, which include a lot of fast food and processed meals with greater sugar content in daily diets. The Centers for Disease Control and Prevention (CDC) released the National Diabetics Statistics Report (2020), which states that 10.5% of adults in the USA have diabetes and 45.8% of adults are obese [25].Two forms of diabetes (types 1 and 2) can be distinguished based on the etio-pathogenesis. While type 2 diabetes, which is the most prevalent kind, involves resistance to insulin, type 1 diabetes is defined by the death of pancreatic B cells as a result of the body's autoimmune reaction, which results in an insulin shortage [26].Diabetes causes persistent hyperglycemia, which can develop in retinopathy, neuropathy, nephropathy, peripheral vascular, cerebrovascular, and ischemic heart disorders [27, 28, 29].Nevertheless the body's inflammatory reactions and degree of oxidative stress are
Crucial in the development of the issues mentioned above.
Reduced endothelial NO production and bioavailability, elevated plasma glucose concentration, free fatty acid, homocysteine, and methylarginines are the main symptoms of non-insulin-dependent diabetes. [30] Several studies indicate that NO affects human hemodynamics, insulin sensitivity, and energy substrate oxidation regulation
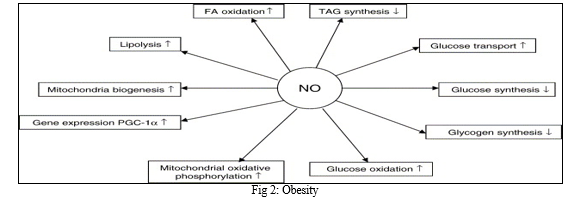
The overall impact of physiological NO levels on the metabolism of energy substrates. Nitric oxide inhibits GPAT activity, which lowers TAG production and promotes fatty acid oxidation by decreasing malonyl-CoA availability. Furthermore, NO mediates leptin-stimulated lipolysis and increases basal lipolysis. In addition, nitric oxide promotes glucose transport in adipose and muscular tissue, raises glucose oxidation, and lowers the production of glycogen in the liver. Additionally, recent research demonstrated that NO promotes mitochondrial biogenesis by triggering the production of PGC-1α.
It has been shown through the findings of numerous in vivo and in vitro investigations that feeding l-arginine to diabetic and obese rats lowers their blood glucose levels [31,32,33].Furthermore, in hyper cholesterol patients [36] and animal models [30,34,35], l-arginine increased vascular reactivity. Considering the health benefits of l-arginine, it ought to be consumed to reduce the risk of obesity and diabetes. But l-arginine consumption directly caused gastrointestinal issues, thus l-arginine-enriched meals have taken over as the preferred option. Watermelon consumption raised plasma l-arginine levels substantially. Wu et al. [9] reported that adding watermelon juice to Zucker diabetic fatty rats—an animal model commonly used to study non-insulin dependent diabetes—increased the levels of l-arginine and reduced those of glucose, homocysteine, free fatty acid, and methylarginines(9).Conversely, it increased tetrahydrobiopterin levels in the heart and acetylcholine-mediated vascular relaxation as well as the activity of GTP cyclohydroxylase-1.Furthermore, based on their findings in animal models, Wu et al. [9] suggested consuming watermelon juice as a functional meal to fight obesity and diabetes.
When given orally to human beings, watermelon juice can serve as a useful substitute for arginine supplements. Watermelon juice improved human immunological function and cardiovascular health while also efficiently regulating the body's metabolism. In a research by Figueroa et al. [31], watermelon consumption improved vascular function and decreased carotid wave reflection, brachial blood pressure, and ankle blood pressure in obese middle-aged adults with pre-hypertension. In a similar vein, eating watermelon caused obese persons to feel fuller and saw reductions in their body weight, body mass index (BMI), and waist to hip ratio. Additionally, compared to traditional refined carbohydrate snacks, Lum et al. [32] revealed that watermelon can effectively suppress hunger and support weight management.
C. Anti-Ulcerative Colitis Property Of Watermelon
One of the common inflammatory bowel diseases that inflames the mucosa across the gastrointestinal tract is ulcerative colitis [33]. The breakdown of goblet cells, crypts, and ulcer formation are hallmarks of ulcerative colitis [34].Furthermore, colon cancer, the second most widespread disease with the highest death rate, can arise from ulcerative colitis in its chronic phases [35].In addition to colon cancer, ulcerative colitis is linked to the
beginning of other related conditions such psoriasis, ankylosing spondylitis, and rheumatoid arthritis [36].One sign of ulcerative colitis is a decrease in the absorption of l-arginine by colonocyte [37, 38].The ulcerative colitis patients showed lower levels of l-arginine, which affected the colon's histology and normal mucosal permeability, as reported by Coburn et al. [39].Additionally, l-arginine supplementation reduced pro-inflammatory cytokine levels, improved several related clinical parameters, and enhanced antioxidant activity to relieve ulcerative colitis symptoms. [40, 41].The watermelon's high concentration of l-citrulline, which is a precursor to l-arginine, has potential therapeutic benefits for ulcerative colitis. A recent study by Hong et al. [22] showed that the watermelon supplementation induced improvements in cellular kinetics, endogenous nitric oxide levels, and the micro-architecture of colon crypts. According to Hong et al.'s [22] theory, watermelon boosts NO levels which in turn would synergistically promote the expression of peroxisome proliferator-activated receptor–γ (PPAR–γ), which would reduce oxidative stress and inflammation. Excess of reactive oxygen species production causes oxidative stress, which is fatal to the macromolecular components of the cells and damages DNA.
This is one of the main factors that worsens the pathogenesis of ulcerative colitis. [42]Specifically, DNA damage from oxidative stress promotes the development of cancer. For example, the down-regulation of tumor suppressor genes is facilitated by the production of 8-hydroxydeoxyguanosine (8-OHdG), an oxidized derivative of deoxyguanosine generated by the interaction between reactive hydroxyl radical and DNA nucleobase [43,44].Rats given DSS had higher than normal amounts of 8-OHdG, according to Hong et al. [22], but the 8-OHdG content decreased when watermelon was added. The watermelon's high antioxidant profile may have contributed to the reduction of 8-OHdG levels by reducing oxidative stress and shielding DNA from harm.
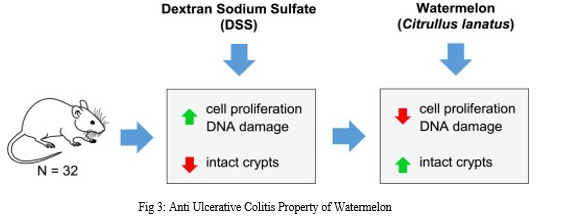
This study was designed to find out how watermelon affected rats receiving a high-fat diet and Dextran Sodium Sulfate (DSS) for colitis. Our hypothesis was that watermelon, through controlling cell homeostasis and preserving the quantity of intact crypts, would lessen the severity of colitis.
The control diet, control diet + DSS, 0.33% watermelon powder diet, and 0.33% watermelon powder diet + DSS were the four groups into which the forty rats were split. Following four weeks of specified feeding, rats in the DSS group were given 3% (w/v) DSS (40 kDa) in their drinking water for two days. Administration of DSS reduced cell differentiation and the amount of intact colonic crypts, but enhanced colonocyte proliferation and death (P?<?.05).
The application of watermelon therapy eliminated the impact of DSS on cell proliferation and decreased the loss of colonic crypts (P?<?.05. Cyclin D1's mRNA expression was elevated by DSS, however this impact was mitigated by treatment with watermelon. These findings show that supplementing with watermelon reduces colitis by preserving the typical architecture of the colonic crypt and regulating the balance between cell division and death.
D. Antioxident Properties Of Watermelon Seeds
Classified into phenolic acids, flavonoids, lignans, and stilbenes, polyphenolic substances are essential antioxidants. There are two hydroxyl groups at least joined to an aromatic ring in the structure of polyphenols. Polyphenolic chemicals are primarily responsible for the antioxidant qualities found in fruits and vegetables. It has been observed by Tilili et al. that the hydrophilic antioxidant activity of watermelon is due to the presence of polyphenols. The amount of polyphenols in fresh watermelon juice ranges from 16.94 to 20.23 mg / 100 mL. This means that consuming watermelon as a snack or a beverage might increase the body's antioxidant capacity and enhance biological processes like adhesion and cell signaling as well as additional biological processes. However, the antioxidant potentials are determined by the polyphenols' structure, amount, absorption, and bioavailability. The watermelon has a variety of polyphenols, and only a small number of research have sought to determine their precise composition (Table 1). Abu-Reidah et al. used high-performance liquid chromatography in conjunction with electrospray ionization–quadrupole time-of-flight mass spectrometry (HPLC–ESI–QTOF–MS) to evaluate the phytochemicals found in the methanolic extracts of watermelon. [46]
E. Anticancer Properties Of Watermelon Seeds
Cancer is a terrible illness that kills a lot of people in a lot of countries all over the world. The molecular mechanisms behind carcinogenesis in biological systems can be influenced by the correlation seen between the active components of food and gene expression in many metabolic pathways. For example, the active ingredient in watermelon, lycopene, can reduce the development of cancer by acting against tumor spreading and by inhibiting DNA mutation. Nahum et al report that lycopene causes modifications to the cell cycle machinery, namely by inhibiting the G1 phase in endometrial and breast cancers in humans. [46]In cancer cells, cyclin-dependent kinase (CDK) 1 and 3 activities were decreased by lycopene injection. Additionally, lycopene's antioxidant capacity decreases. Furthermore, lycopene's antioxidant qualities lessen oxidative stress and support the anti-proliferative actions against malignant cells. Lycopene has been shown in several studies to have anti-cancerous properties both in vitro and in vivo.
The detailed molecular reasoning underlying the lycopene-mediated control of gene interaction is still being investigated, though [47]. Colon cancer ranks second among the malignancies that kill people most often in the globe. An imbalance between the planned cell death (apoptosis) and growth of cells can lead to colon cancer. But according to research, the majority of colon cancers may be avoided with the right dietary changes. While rats with colon cancer showed a reduction in cell proliferation when given watermelon supplements, there was no discernible effect on apoptosis. The presence of copious l-citrulline and its role in the generation of endothelial nitric oxide (NO) might be the key component contributing to the watermelon's tumoricidal properties. The addition of watermelon powder to male Sprague-Dawleys' meals, according to Glen et al. Watermelon powder was added to the meals of male Sprague-Dawley rats that had been given a colon cancer inducer in order to lower the chances of aberrant crypt foci development by lowering DNA inflammation and oxidative damage. Furthermore, the supplementation of watermelon additionally altered the expression of DNA repair enzymes to battle the cancer, and an increase in endogenous nitric oxide synthesis added to the relief of carcinogenic effects.
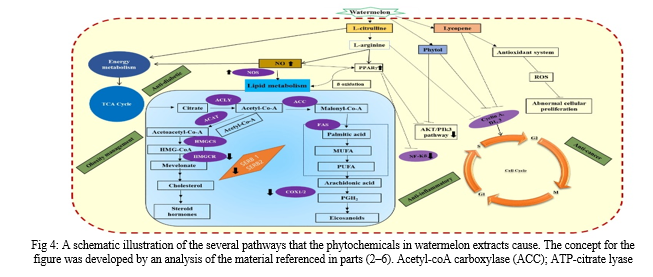
(ACLY); COX1/2, cyclooxygenase; Akt, alpha serine-threonine protein kinase; HMGCS, 3-hydroxy-3-methylglutaryl-CoA synthase; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; FAS, fatty acid synthase; MUFA, monounsaturated fatty acid; NO, endogenous nitric oxide; NOS, nitric oxide synthase; PPAR-γ, peroxisome proliferator-activated receptor–γ; PUFA, polyunsaturated fatty acid; PGH2, prostaglandin-H2; ROS, reactive oxygen species; SERB, sterol regulatory element binding proteins; TCA, tricarboxylic acetic acid cycle.[50]. The two most common malignancies in women with the highest death rates are breast and cervical cancers. Studies have been conducted on breast and cervical cancer cell lines to examine the anti-proliferative properties of watermelon leaf extracts [49]. Six watermelon cultivars' worth of leaf extracts were examined on cell lines representing cervical cancer (C33A, HeLa, and SiHa) and breast adenocarcinoma (MCA-MB-231 and MCF-7) [49]. The watermelon leaf extracts' ability to inhibit the proliferation of both cancer cell lines as compared to normal cells was demonstrated by the in vitro MTT test and microscopic examination of the cells. On the other hand, the C33A cervical cancer cell line demonstrated a significant level of sensitivity to the extracts. Microscopic examinations of the cancer cell lines clarified the decrease in cell quantity and cellular cell size in MCF-7, MDA-MB-231, and C33A lines [49]. The study also proposed that watermelon leaf extracts had anti-cancer properties that vary depending on the cultivar.
F. Additional Benefits Of Watermelon Seeds
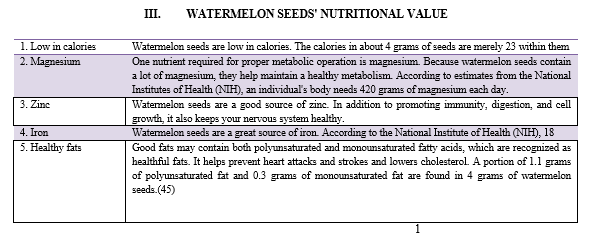
- Benefits of Watermelon Seed for Health: Watermelon seeds can benefit our bodies in unexpected ways since they are a rich source of proteins, essential fatty acids, magnesium, and zinc. The following are a few of the noteworthy benefits:
- Enhances the Condition of the Skin: The sprouted seeds from watermelon, high in vitamin C and other antioxidants, help to exfoliate your skin. Its oil is commonly used in many cosmetic products to treat acne and the signs of aging beginning. Watermelon seeds are a great source of magnesium, which improves the appearance of your skin overall. Disorders like eczema and other dry, itchy skin issues can benefit from it. Watermelon seeds will moisturize your dull, dry skin. The zinc found in seeds has the ability to accelerate protein synthesis, cell division, and repair, which can delay the aging process.
- Promotes the Regrowth of Hair: Everyone values having well-maintained hair. So why not use watermelon seeds to achieve this? Watermelon seeds are rich in proteins, iron, magnesium, zinc, and copper, all of which have been demonstrated to improve the quality of hair. These seeds promote the growth and fortification of hair. The manganese in the seeds helps to lessen damage and hair loss.
- Strengthen your Immune System: Watermelon seeds contain iron and other minerals that support a stronger immune system. The vitamin B complex that these seeds contain is also helpful in this regard.
- Avoid bone Thinning: If you experience health issues such as weak bones or osteoporosis, consider adding watermelon seeds. If ingested frequently, watermelon seeds—which are high in potassium, magnesium, and copper—can help prevent bone problems.
- Encourages the Neurological System: Watermelon seeds are a great source of vitamin B, which is important for the health of your nervous system and brain. It can help with both mood problems and dementia. Watermelon is a great source of zinc, which is essential for the male reproductive system to function properly. Zinc has the ability to improve sperm quality. [45]
Conclusion
Watermelon is a rich source of phytochemicals with significant medicinal value, most of which are linked to the high concentrations of polyphenols, lycopenes, and citrulline. Supplementing with watermelon extracts helps patients recover from a variety of pathological conditions as well as from the unavoidable adverse consequences of those conditions. Watermelon tissues, including seeds, and fruit contain secondary metabolites with nutraceutical potential that act on many potential therapeutic targets related to illnesses including diabetes, cancer, inflammation, and obesity. To increase the use of phytochemicals in the nutraceutical sectors, further research on the extraction of the active phytochemical and the molecular regulatory mechanisms of the bioactive substances in watermelon are necessary. Furthermore, the assessment of phytochemical and pharmacokinetic data of essential secondary metabolites in watermelons can speed up the process of creating drugs to treat terrible illnesses. Furthermore, consuming watermelon as a functional diet might delay the beginning of a number of human illnesses. When combined, the knowledge gathered about the phytochemicals found in fruits and vegetables can be a useful tool in hastening the development of several plant-based medication candidates with low side effects.
References
[1] Assefa A.D., Hur O.S., Ro N.Y., Lee J.E., Hwang A.J., Kim B.S., Rhee J.H., Yi J.Y., Kim J.H., Lee H.S., et al. Fruit Morphology, Citrulline, and Arginine Levels in Diverse Watermelon (Citrullus lanatus) Germplasm Collections. Plants. 2020;9:1054. doi: 10.3390/plants9091054. [2] U.S. Department of Agriculture ARS . In: USDA National Nutrient Database for Standard Reference, Release 27. Service A.R., editor. Department of Agriculture; Washington, DC, USA: 2015. [3] Rimando A.M., Perkins-Veazie P.M. Determination of citrulline in watermelon rind. J. Chromatogr. A. 2005;1078:196–200. doi: 10.1016/j.chroma.2005.05.009. [4] Rao A.V., Agarwal S. Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr. 2000;19:563–569. doi: 10.1080/07315724.2000.10718953 [5] Romero M.J., Platt D.H., Caldwell R.B., Caldwell R.W. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc. Drug Rev. 2006;24:275–290. doi: 10.1111/j.1527-3466.2006.00275.x. [6] Tlili I., Hdider C., Lenucci M.S., Riadh I., Jebari H., Dalessandro G. Bioactive compounds and antioxidant activities of different watermelon (Citrullus lanatus (Thunb.) Mansfeld) cultivars as affected by fruit sampling area. J. Food Compos. Anal. 2011;24:307–314. doi: 10.1016/j.jfca.2010.06.005 [7] Tomes M.L., Johnson K.W., Hess M. The carotene pigment content of certain red fleshed watermelons. Inproc. Am. Soc. Hortic. Sci. 1963;82:460–464. [8] Wada M. Uber Citrullin, eineneueAminosaureimPreBsaft der Wassermelone, Citrullus vulgaris schrad. BiochemischeZeitschrift. 1930;224:420–429. [9] Wu G., Collins J.K., Perkins-Veazie P., Siddiq M., Dolan K.D., Kelly K.A., Heaps C.L., Meininger C.J. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J. Nutr. 2007;137:2680–2685. doi: 10.1093/jn/137.12.2680 [10] Kaul P. Nutritional potential, bioaccessibility of minerals and functionality of watermelon (Citrullus vulgaris) seeds. LWT-Food Sci. Technol. 2011;44:1821–1826 [11] Suliburska J., Bogdanski P., Krejpcio Z., Pupek-Musialik D., Jablecka A. The effects of L-arginine, alone and combined with vitamin C, on mineral status in relation to its antidiabetic, anti-inflammatory, and antioxidant properties in male rats on a high-fat diet. Biol. Trace Elem. Res. 2014;157:67–74. doi: 10.1007/s12011-013-98675 [12] Alam M.A., Kauter K., Withers K., Sernia C., Brown L. Chronic L-arginine treatment improves metabolic, cardiovascular and liver complications in diet-induced obesity in rats. Food Funct. 2013;4:83–91. doi: 10.1039/C2FO30096F. [13] Evans R.W., Fernstrom J.D., Thompson J., Morris S.M., Jr., Kuller L.H. Biochemical responses of healthy subjects during dietary supplementation with L-arginine. J. Nutr. Biochem. 2004;15:534–539. doi: 10.1016/j.jnutbio.2004.03.005. [14] Wu G., Meininger C.J. Arginine nutrition and cardiovascular function. J. Nutr. 2000;130:2626–2629. doi: 10.1093/jn/130.11.2626. [15] Hong M.Y., Hartig N., Kaufman K., Hooshmand S., Figueroa A., Kern M. Watermelon consumption improves inflammation and antioxidant capacity in rats fed an atherogenic diet. Nutr. Res. 2015;35:251–258. doi: 10.1016/j.nutres.2014.12.005 [16] Jobgen W.S., Fried S.K., Fu W.J., Meininger C.J., Wu G. Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [17] Jobgen W., Fu W.J., Gao H., Li P., Meininger C.J., Smith S.B., Spencer T.E., Wu G. High fat feeding and dietary L-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids. 2009;37:187–198. doi: 10.1007/s00726-009-0246-7. [18] Jensen-Urstad A.P., Semenkovich C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2012;1821:747–753. doi: 10.1016/j.bbalip.2011.09.017 [19] Baenke F., Peck B., Miess H., Schulze A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Models Mech. 2013;6:1353–1363. doi: 10.1242/dmm.011338. [20] Iantorno M., Campia U., Di Daniele N., Nistico S., Forleo G.B., Cardillo C., Tesauro M. Obesity, inflammation and endothelial dysfunction. J. Biol. Regul. Homeost. Agents. 2014;28:169–176 [21] Zaiss A.K., Zuber J., Chu C., Machado H.B., Jiao J., Catapang A.B., Ishikawa T.O., Gil J.S., Lowe S.W., Herschman H.R. Reversible suppression of cyclooxygenase 2 (COX-2) expression in vivo by inducible RNA interference. PLoS ONE. 2014;9:e101263. doi: 10.1371/journal.pone.0101263. [22] Hong M.Y., Tseng Y.T., Kalaba M., Beidler J. Effects of watermelon powder supplementation on colitis in high-fat diet-fed and dextran sodium sulfate-treated rats. J. Funct. Foods. 2019;54:520–528. doi: 10.1016/j.jff.2019.02.005 [23] Shanely R.A., Zwetsloot J.J., Jurrissen T.J., Hannan L.C., Zwetsloot K.A., Needle A.R., Bishop A.E., Wu G., Perkins-Veazie P. Daily watermelon consumption decreases plasma sVCAM-1 levels in overweight and obese postmenopausal women. Nutr. Res. 2020;76:9–19. doi: 10.1016/j.nutres.2020.02.005 [24] Connolly M., Lum T., Marx A., Hooshmand S., Kern M., Liu C., Hong M.Y. Effect of Fresh Watermelon Consumption on Risk Factors for Cardiovascular Disease in Overweight and Obese Adults (P06-102-19) Curr. Dev. Nutr. 2019;3 doi: 10.1093/cdn/nzz031.P06-102-19 [25] Center for Disease Control and Prevention . National Diabetes Statistics Report. Centers for disease control and prevention, US Department of Health and Human Services; Atlanta, GA, USA: 2020. [26] Johnson J.A., Pohar S.L., Majumdar S.R. Health care use and costs in the decade after identification of type 1 and type 2 diabetes: A population-based study. Diabetes Care. 2006;29:2403–2408. doi: 10.2337/dc06-0735 [27] Kahn R., Buse J., Ferrannini E., Stern M. The metabolic syndrome: Time for a critical appraisal. Diabetologia. 2005;148:1684–1699. doi: 10.1007/s00125-005-1876-2 [28] Inzucchi S.E., Matthews D.R., Buse J.B., Diamant M., Ferrannini E., Nauck M., Peters A.L., Tsapas A., Wender R., Matthews D.R. Response to Comments on Inzucchi et al. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach. Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. [29] Marliss E.B., Chevalier S., Gougeon R., Morais J.A., Lamarche M., Adegoke O.A., Wu G. Elevations of plasma methylarginines in obesity and ageing are related to insulin sensitivity and rates of protein turnover. Diabetologia. 2006;49:351–359. doi: 10.1007/s00125-005-0066-6. [30] Creager M.A., Gallagher S.J., Girerd X.J., Coleman S.M., Dzau V.J., Cooke J.P. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J. Clin. Investig. 1992;90:1248–1253. doi: 10.1172/JCI115987. [31] Figueroa A., Sanchez-Gonzalez M.A., Perkins-Veazie P.M., Arjmandi B.H. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: A pilot study. Am. J. Hypertens. 2011;24:40–44. doi: 10.1038/ajh.2010.142. [32] Lum T., Connolly M., Marx A., Beidler J., Hooshmand S., Kern M., Liu C., Hong M.Y. Effects of fresh watermelon consumption on the acute satiety response and cardiometabolic risk factors in overweight and obese adults. Nutrients. 2019;11:595. doi: 10.3390/nu1103059 [33] Machiels K., Joossens M., Sabino J., De Preter V., Arijs I., Ballet V., Claes K., Verhaegen J., Van Assche G., Rutgeerts P.J., et al. 187 Bacterial Dysbiosis in Ulcerative Colitis Patients Differs From Crohn’s Disease Patients. Gastroenterology. 2012;142:S-46. doi: 10.1016/S0016-5085(12)60176-0. [34] Danese S., Fiocchi C. Ulcerative colitis. N. Engl. J. Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [35] Clapper M.L., Cooper H.S., Chang W.-C.L. Dextran sulfate sodium-induced colitis-associated neoplasia: A promising model for the development of chemopreventive interventions 1. Acta Pharm. Sin. 2007;28:1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [36] Ghosh S., Mitchell R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J. Crohn’s Colitis. 2007;1:10–20. doi: 10.1016/j.crohns.2007.06.005. [37] Hatoum O.A., Binion D.G., Otterson M.F., Gutterman D.D. Acquired microvascular dysfunction in inflammatory bowel disease: Loss of nitric oxide-mediated vasodilation. Gastroenterology. 2003;125:58–69. doi: 10.1016/S0016-5085(03)00699-1 [38] Hong S.K., Maltz B.E., Coburn L.A., Slaughter J.C., Chaturvedi R., Schwartz D.A., Wilson K.T. Increased serum levels of L-arginine in ulcerative colitis and correlation with disease severity. Inflamm. Bowel Dis. 2010;16:105–111. doi: 10.1002/ibd.21035. [39] Coburn L.A., Horst S.N., Allaman M.M., Brown C.T., Williams C.S., Hodges M.E., Druce J.P., Beaulieu D.B., Schwartz D.A., Wilson K.T. L-arginine availability and metabolism is altered in ulcerative colitis. Inflamm. Bowel Dis. 2016;22:1847–1858. doi: 10.1097/MIB.0000000000000790. [40] Ren W., Yin J., Wu M., Liu G., Yang G., Xion Y., Su D., Wu L., Li T., Chen S., et al. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS ONE. 2014;9:e88335. doi: 10.1371/journal.pone.0088335. [41] Coburn L.A., Gong X., Singh K., Asim M., Scull B.P., Allaman M.M., Williams C.S., Rosen M.J., Washington M.K., Barry D.P., et al. L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS ONE. 2012;7:e33546. doi: 10.1371/journal.pone.0033546. [42] Roessner A., Kuester D., Malfertheiner P., Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol. Res. Pract. 2008;204:511–524. doi: 10.1016/j.prp.2008.04.011. [43] Valavanidis A., Vlachogianni T., Fiotakis C. 8-hydroxy-2?-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health. 2009; 27:120–139. doi: 10.1080/10590500902885684 [44] Yasui M., Kanemaru Y., Kamoshita N., Suzuki T., Arakawa T., Honma M. Tracing the fates of site-specifically introduced DNA adducts in the human genome. DNA Repair. 2014; 15:11–20. doi: 10.1016/j.dnarep.2014.01.003. [45] Ijfans International Journal Of Food And Nutritional Sciencesin- Discussion Of The Nutritional Components And Properties Of Watermelon Seeds Margret Chandira Rajappa*1, Lokeshwaran Sekar*2, Devisowndarya Nagendra Boopathy*2, Ajithkannan Radhakrishnan*3, Dhanavel Venkatachalapathi*4 Vinayaka Missions College Of Pharmacy, Vinayaka Missions Research Foundation (DU), Yercardadivaram,Salem,Tamil Nadu, INDIA [46] First Report On Laxative Activity Of Citrullus Lanatus S Sharma, P Sarvesh, J Dwivedi, T Amita - Pharmacologyonline, 2011 [47] Nahum, A.; Hirsch, K.; Danilenko, M.; Watts, C.K.; Prall, O.W.; Levy, J.; Sharoni, Y. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27 Kip1 in the cyclin E–cdk2 complexes. Oncogene 2001, 20, 3428–3436. [48] Glenn, K.; Klarich, D.S.; Kalaba, M.; Figueroa, A.; Hooshmand, S.; Kern, M.; Hong, M.Y. Effects of Watermelon Powder and L-arginine Supplementation on Azoxymethane-Induced Colon Carcinogenesis in Rats. Nutr. Cancer 2018, 70, 938–945 [49] Sueakham, T.; Chantaramanee, C.; Iawsipo, P. Anti-proliferative effect of Thai watermelon leaf extracts on cervical and breast cancer cells. NU. Int. J. Sci. 2018, 15, 89–95. [Google Scholar] [50] doi https://www.mdpi.com/1420-3049/25/22/5258#B99-molecules-25-05258 [51] Perkins-Veazie, P.; Davis, A.; Collins, J.K. Watermelon: From dessert to functional food. Isr. J. Plant Sci. 2012, 60, 395–402.
Copyright
Copyright © 2024 Ms. Aarti Labhane, Laiba Momin, Raj Patil, Ms. Reema Rani, Dr. Rupali Tasgaonkar, Sonal Nuche, Omkar Mohite. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET59308
Publish Date : 2024-03-22
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

