Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Identification of Urinary Tract Infection Causing Bacteria from Microbial Colony Images using Deep Learning
Authors: Dr. D. R. Ramesh Babu, Sunanda , Ayush Kumar Manas, Dhruv Rathi, Tejas R, Tushar Pathak
DOI Link: https://doi.org/10.22214/ijraset.2024.59944
Certificate: View Certificate
Abstract
Diagnosis of microbiological and bacterial infections involves extensive procedures of sample culture and microscopic examination. The diagnosis process begins with the identification of symptoms followed by collection of test samples in the form of blood, scraps of skin lesions, mucus, sputum, urine etc. The entire process of bacteria routine culture and bacteriological examination may take as long as 10 to 11 days. Due to the long time required for the standard process of species identification, high costs and need of human expertise it is beneficial to use methods that do not rely on conventional methods. In this work we propose a comprehensive Deep Learning based approach to identify urinary Tract Infection (UTI) causing bacteria from microbial colony images of bacteria cultured on agar plates. The approach explains the use of VGG-19 and Inception-v3 deep learning architectures, in bacteria identification evaluated on The Annotated Germs for Automated Recognition (AGAR) dataset, an image dataset of microbial colonies cultured on agar plates. The findings affirmed the significant potential of employing deep learning techniques for the identification of microbial colonies and their classification using Petri dish images.
Introduction
I. INTRODUCTION
Urinary tract infections (UTIs) stand as one of the most prevalent bacterial infections affecting millions worldwide. Characterized by the invasion of bacteria into the urinary system, UTIs pose as a significant health burden. The causative agents, often bacteria like Escherichia coli and even fungi, can cause inflammation in various parts of the urinary tract, causing discomfort, pain, and, if left untreated, potential complications such as kidney infections. UTIs not only diminish the quality of life for affected individuals but also strain healthcare systems globally. The ubiquity and recurrent nature of UTIs underscore the pressing need for efficient diagnostic methods to ensure timely and accurate intervention [4].
The traditional diagnostic path for identifying the bacteria responsible for UTIs is complex. It starts with identifying symptoms, which can range from frequent and painful urination to lower abdominal pain. When a UTI is suspected, samples are collected, most commonly in the form of urine. However, the following steps involve routine culture and bacteriological examination, which is a time-consuming and resource-intensive process. Culturing bacteria from collected samples and analysing them under a microscope can take 10 to 11 days, delaying diagnosis and impeding treatment initiation. In addition, this procedure necessitates a team of proficient microbiologists, which adds to the difficulty and expense of diagnosing UTIs . One of the biggest barriers to providing timely and efficient healthcare is the length of time needed for traditional species identification procedures for bacteria that cause UTIs [1]. This issue is made worse by the resource-intensive nature of these methods and the scarcity of skilled labour. It becomes essential to find alternative approaches that can overcome the limitations of traditional diagnostic techniques. The difficulties presented by current UTI diagnostic methodology necessitate the investigation of novel approaches to address the critical issues of time inefficiency, high costs, and reliance on skilled human personnel. The financial burden of extensive culture and examination procedures necessitates the development of cost-effective alternatives that can streamline the diagnostic process without sacrificing accuracy. The intersection of medical science and advanced technologies, particularly deep learning, offers a promising avenue in this context. The search for a more efficient and accessible diagnostic solution for UTIs is part of a larger effort to improve global healthcare outcomes and reduce the burden of preventable complications associated with bacterial infections.
This project's main goal is to develop and put into practice a Deep Learning-based method for the quick and precise identification of bacteria that cause urinary tract infections [2]. This method aims to lower the time, expense, and reliance on human skill associated with diagnosis. The project employs The Annotated Germs for Automated Recognition (AGAR) dataset, a collection of microscopic images capturing microbial colonies cultured on agar plates. This dataset ensures the representation of diverse bacterial species relevant to UTIs. In particular, the research uses VGG-19 and Inception-v3, two cutting-edge deep learning architectures, for bacteria identification.
II. LITERATURE SURVEY
|
Paper title |
Methodology used |
Merits |
Demerits |
|
Deep Neural Networks Approach to Microbial Colony Detection—A Comparative AnalysisSylwia Majchrowska1,2, JaroslawPawlowski1,2, Natalia Czerep1,3, AleksanderG´orecki1,2, Jakub Kuci´nski1,3, and Tomasz Golan1 |
Three different architectures were adopted and tested on a high resolution subset of AGAR dataset. One stage YOLOv4 model obtained best results. |
Results achieved serve as benchmark for future Experiments. |
Need to collect large amounts of balanced data |
|
Identifying Bacteria Species on Microscopic Polyculture Images Using Deep Learning Adriana Borowa, Dawid Rymarczyk, Dorota Ocho´nska |
Pipeine based approach which returns labels for the images corresponding to bacteria species. |
Introduces a mechanism that allows for multi label classification without a decrease in interpretability. |
Image magnification is necessary for the dataset images which is an overhead. |
|
AGAR a microbial colony dataset for deep learning detection Sylwia Majchrowska1,2, Jaros?aw Paw?owski1,2, Grzegorz Gu?a1,3, Tomasz Bonus1, Agata Hanas1, Adam Loch1 |
Two architectures were used. Faster R-CNN and Cascade R-CNN with 4 backbones: ResNet-50, ResNet-1010, ResNeXt-101 and HRNet |
Highly accurate results due to good image quality in AGAR Dataset. |
There is no particular demerit as such but still accuracy needs to be improved more. |
||||
|
Automatic Identification of Single Bacterial Colonies Using Deep and Transfer Learning SHIMAA A. NAGRO1, MOHAMMED A. |
Transfer learning to initialize model weights. Other architectures employed were ResNet-18, AlexNet, VGG-16, SqueezeNet and DenseNet |
High accuracy obtained from transfer learning and VGG-16 Architecture. |
Preprocessing required to induce variety in triangle and testing data. |
|
|||
|
Machine Learning and Deep Learning Based Computational Approaches in Automatic Microorganisms Image Recognition: Methodologies, Challenges and Development Priya Rani1 · Shallu Kotwal2 |
Review of cutting edge architectures explored, in the the field of bacteria classification using deep learning. |
The review covers various aspects, including image pre-processing, feature extraction, classification techniques, and evaluation measures, providing a holistic understanding of the methodologies used. |
The paper mentions investigating methodological limitations. It would be crucial to understand the limitations to ensure the reliability and generalizability of the proposed approaches. |
|
|||
|
Automated Bacterial Classifications Using Machine Learning Based Computational Techniques. Shallu Kotwal1 · Priya Rani2 · Tasleem Arif1 · |
Review of conventional and unconventional methods used in the area of research of bacteria classification. |
Machine learning methods, with the aid of artificial intelligence techniques, have shown tremendous performance in automatically detecting bacteria. This could lead to faster and more accurate results compared to traditional methods. |
Discussing ethical considerations such as privacy, consent, and potential societal impacts is important in sensitive domains like healthcare. |
|
|||
|
Deep Convolutional Neural Network for Microscopic Bacteria Image Classification Md. Ferdous Wahid; Md. Jahid Hasan; Md. Shahin Alom |
CNN and ‘Inception’ architecture based on transfer learning to classify chosen 7 varieties of bacteria. |
The use of machine learning, specifically deep convolutional neural networks (CNNs) based on the Inception architecture, automates the process of recognizing and classifying bacteria from microscopic images. |
The dataset, consisting of seven varieties of bacteria, might not represent the full spectrum in real-world scenarios. This could lead to biased results. |
|
|||
|
An Enhanced Classification of Bacteria Pathogen on Microscopy Images Using Deep Learning Son Ali Akbar; Kamarul Hawari Ghazali; Habsah Hasan; Zeehaida |
DensNet201 pre-trained CNN architecture has been used for deep feature extraction and classification. In addition, the transfer learning with the freeze layer technique applied can enhance the accuracy performance and reduce the false-positive rate. |
The application of transfer learning with the freeze layer technique is a strategic choice. Leveraging a pre-trained model like DensNet201 allows the model to benefit from knowledge learned on a large dataset, even when dealing with a smaller dataset for bacterial pathogen classification. |
While transfer learning is a powerful approach, it might not always transfer well to the specific domain of bacterial pathogen classification. |
|
|||
III. AGAR DATASET
The dataset comprises of 18,000 photos with 336,442 annotated colonies of 5 standard microbes. Notably, the dataset exhibits a balanced distribution of instances for different microbes, crucial for building robust deep learning models [13].
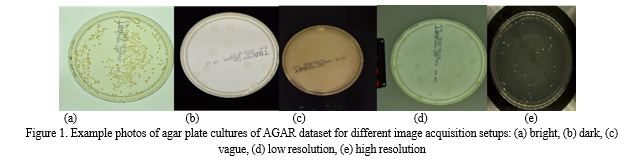
The dataset includes five classes, namely Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli and Candida albicans, while annotations are stored in JSON format with information about the number and type of microbe, environment, and coordinates of bounding boxes. The dataset consists of higher and lower-resolution images. The higher-resolution images are further classified into bright, dark, and vague subgroups which are a result of variations in lighting conditions during data acquisition as seen in Figure 1. This enhances the dataset's versatility and ensures its effectiveness across different experimental setups. This balance is crucial for the development of a resilient deep learning model. The AGAR dataset was chosen for this work keeping in mind the most common bacteria which cause urinary tract infections. Even though there are some bacteria like Klebsiella pneumoniae, Proteus mirabilis which might present themselves as more infectious than the ones in the AGAR dataset, the AGAR dataset is the closest we can get, to satisfy the suitable conditions for our work [3].
A. Types of Images
IV. IMPLEMENTATION
In order to identify the bacteria, the first step was to identify microbial colonies. To achieve this, simple image segmentation was performed on the images.
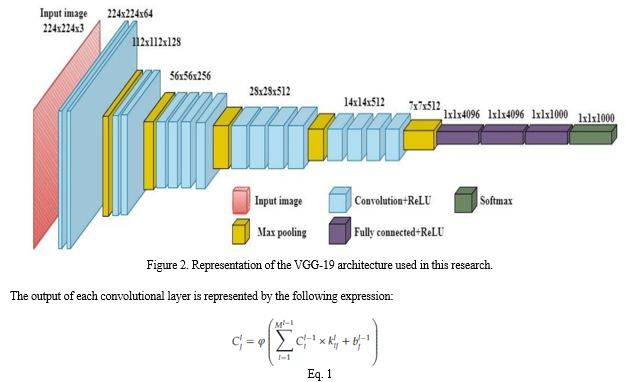
Upon image segmentation, an ensemble of two deep learning architectures was applied on the dataset namely VGG19 (Visual Geometry Group) and Inception-v3 (Iv3). The VGG-19 is a Convolutional Neural Network that is 19 layers deep (16 convolution layers, 3 Fully connected layer, 5 MaxPool layers and 1 SoftMax layer) as seen in Figure 2. The network takes in RGB images as input of size (224X224) pixels. The top layer used in VGG-19 is trained on ImageNet weights. The pretrained top layer is capable of classifying images of upto 1000 different categories [14].
In which × is the convolutional function which describes the connection between the weights of the ith and jth features in the (l−1)th, lth layers, bj is the bias value, and φ is the activation function [15].
Inception-v3 is convolutional neural network that is 48 layers deep. It takes in RGB images of size 299x299 pixels as input. In the case of Inception-v3 however, the top layer with ImageNet weights was omitted owing to different experimental observations of induces bias. However, the exclusion of the top layer forces a trade-off between the induced bias and decreasing variance [16].
Despite the availability of more efficient, scalable and robust image recognition architectures like Inception-v5, EfficientNet, and Exception Model, the architectures VGG-19 and Inception-v3 were used owing to their efficiency in region specific convolution output, based on modified CNN architecture as seen in Figure 3. EfficientNet is a recognition specific architecture and Exception model is the latest general and highly accurate model. These architectures were not chosen as they did not adhere entirely to the critical aspects of the experiment. As the bacteria colony detection was critical in the classification process, segmentation and models which concentrate on region specific output were chosen. Another reason for choosing Inception-v3 and VGG-19 was their low accuracy loss and suitability for categorical data.
Maxpooling was used in both the individual architectures, as region specific features were needed for colony identification. In the first stage, of training 150 images of each bacterium consisting of both low and high resolution were chosen for model training. The models were individually trained on the data with a split of 80% and 20% for training and validation respectively [5].


Conclusion
In our experiments conducted with the deep architectures VGG-19 and Inception-v3 on the AGAR dataset to classify UTI causing bacteria, we could infer that there is excellent scope for modern deep learning techniques in the field of healthcare, with medical image processing being the greatest example. Both the deep learning architectures performed well in identifying the class of bacteria from images of microbial colonies on petri dishes with high accuracies. Also, the results clearly showcase that an ensemble of two or more such architectures would certainly perform just as well and is better for new data and images of various resolutions. The challenge in bringing out such an approach was the limited availability of image data, other than the AGAR dataset and the manual labelling of random training data. The models require the images to be reasonably clear and of good resolution with ample illumination. Such a requirement would hinder the direct application of these approaches in the real diagnosis process. However, these deep learning techniques are more than capable of identifying abnormal and mixed colonies grown on petri dishes usually small and located at the edge of the dish, which even a trained technician may sometimes overlook. Overall, such automated and technology assisted approaches can help experts in the diagnosis process, and shorten the time required. These methods can be employed in other diagnostic procedures for microbial infections, with the anticipation of yielding favourable outcomes.
References
[1] Shallu Kotwal, et-al.: “Automated Bacterial Classifications Using Machine Learning Based Computational Techniques: Architectures, Challenges and Open Research Issues”, 12 October 2021, https://doi.org/10.1007/s11831-021-09660-0 [2] Priya Rani, et-al.: “Machine Learning and Deep Learning Based Computational Approaches in Automatic Microorganisms Image Recognition Methodologies, Challenges, and Developments”, 31 August 2021, https://doi.org/10.1007/s11831-021-09639-x [3] Sylwia Majchrowska, et-al.: “AGAR a microbial colony dataset for deep learning detection”, 3 Aug 2021, https://arxiv.org/abs/2108.01234 [4] Shimaa A. Nagro, et-al.: “Automatic Identification of Single Bacterial Colonies Using Deep and Transfer Learning”, 14 November 2022, https://ieeexplore.ieee.org/document/9950247 [5] Adriana Borowa, et-al.: “Identifying Bacteria Species on Microscopic Polyculture Images Using Deep Learning”, 26 September 2022, https://ieeexplore.ieee.org/document/9903318 [6] Natalia Czerep, et-al.: “Deep Neural Networks Approach to Microbial Colony Detection—A Comparative Analysis”, 27 July 2022 https://link.springer.com/chapter/10.1007/978-3-031- 11432-8_9 [7] Hedieh Sajedi, et-al.: “Image-processing based taxonomy analysis of bacterial macromorphology using machine-learning models”, 29 August 2020 https://link.springer.com/article/10.1007/s11042-020- 09284-9 [8] Francesco Asnicar, et-al.: “Machine learning for microbiologists”, 15 November 2023, https://link.springer.com/article/10.1038/s41579-023-00984-1 [9] Yuandi Wu, S. Andrew Gadsden, “Machine learning algorithms in microbial classification: a comparative analysis”, 19 October 2023 https://www.frontiersin.org/articles/10.3389/frai.2023.1200994/full [10] Jaken Whipp, et-al.: “YOLO-based Deep Learning to Automated Bacterial Colony Counting”, 29 December 2022, https://ieeexplore.ieee.org/document/9999125 [11] Problem Solving Strategies (Problem Solving in Mathematics) by Arthur Engel [12] Z. Cai and N. Vasconcelos. Cascade r-cnn: Delving into high quality object detection. In 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition, pages 6154–6162, 2018. [13] AGAR Dataset, https://agar.neurosys.com/ [14] Understanding the VGG19 Architecture, https://iq.opengenus.org/vgg19- architecture/ [15] A VGG-19 model with Transfer Learning and Image Segmentation for Classification, https://www.mdpi.com/2624-7402/4/4/56 [16] Inception V3 CNN Architecture Explained, https://medium.com/@AnasBrital98/inception-v3-cnn-architecture-explained- 691cfb7bba08 [17] VGG16 and VGG19, https://keras.io/api/applications/vgg/ [18] InceptonV3, https://keras.io/api/applications/inceptionv3/ [19] T. Shaily and S. Kala, ‘‘Bacterial image classification using convolutional neural networks,’’ in Proc. IEEE 17th India Council Int. Conf. (INDICON), Dec. 2020, pp. 1–6, doi: 10.1109/INDICON49873.2020.9342356. [20] T. Ching and D. S. Himmelstein, ‘‘Opportunities and obstacles for deep learning in biology and medicine,’’ J. Roy. Soc. Interface, vol. 15, no. 141, 2018, Art. no. 20170387. [21] F. Cong, S. Lin, H. Wang, S. Shang, L. Long, R. Hu, Y. Wu, N. Chen, and S. Zhang, ‘‘Biological image analysis using deep learning-based methods: Literature review,’’ Digit. Med., vol. 4, no. 4, p. 157, 2018, doi: 10.4103/digm.digm_16_18. [22] L. Huang and T. Wu, ‘‘Novel neural network application for bacterial colony classification,’’ Theor. Biol. Med. Model., vol. 15, no. 1, pp. 1–16, Dec. 2018, doi: 10.1186/s12976-018-0093-x. [23] H. Sajedi, F. Mohammadipanah, and A. Pashaei, ‘‘Image-processing based taxonomy analysis of bacterial macromorphology using machine-learning models,’’ Multimedia Tools Appl., vol. 79, nos. 43–44, pp. 32711–32730, Nov. 2020, doi: 10.1007/s11042-020-09284-9. [24] H. Wang, H. Ceylan Koydemir, Y. Qiu, B. Bai, Y. Zhang, Y. Jin, S. Tok, E. C. Yilmaz, E. Gumustekin, Y. Rivenson, and A. Ozcan, ‘‘Early detection and classification of live bacteria using time-lapse coherent imaging and deep learning,’’ Light: Sci. Appl., vol. 9, no. 1, p. 118, Dec. 2020, doi: 10.1038/s41377-020-00358-9. [25] B. D. Satoto, M. I. Utoyo, R. Rulaningtyas, and E. B. Koendhori, ‘‘Classification of features shape of Gram-negative bacterial using an extreme learning machine,’’ in Proc. IOP Conf., Earth Environ. Sci., 2020, vol. 524, no. 1, Art. no. 012005, doi: 10.1088/1755-1315/524/1/012005. [26] S. Patel, ‘‘Bacterial colony classification using atrous convolution with transfer learning,’’ Ann. Romanian Soc. Cell Biol., vol. 25, no. 4, pp. 1428–1441, 2021.
Copyright
Copyright © 2024 Dr. D. R. Ramesh Babu, Sunanda , Ayush Kumar Manas, Dhruv Rathi, Tejas R, Tushar Pathak. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET59944
Publish Date : 2024-04-07
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

