Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
Validated Spectrophotometric Method for the Determination of Metalazone in Pure and in its Dosage Form
Authors: Dr P. Suguna
DOI Link: https://doi.org/10.22214/ijraset.2024.63157
Certificate: View Certificate
Abstract
A simple, precise, rapid sensitive and accurate spectrophotometric method have been developed for the estimation of Metalazone in pure form, its formulations. This method is based on oxidative coupling reaction of Metalazone with 1, 10 – Phenanthroline at pH - 4 in the presence of Ferric chloride to form orange red colored product which is extractable at 520 nm. Beer’s law is obeyed in the concentration range 1- 6 ml (10-60 µgml-1). The product obeyed beers law with molar absorptivity of 1.0148x104. Sandal’s sensitivity 0.0359 and RSD was found to be 0.2282 and recovery 96.63%. The method was completely validated and proven to be rugged. The interferences of the ingredients and excipients were not observed. The repeatability and the performance of the proved method were established by point and internal hypothesis and through recovery studies.
Introduction
I. INTRODUCTION
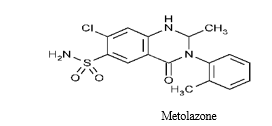
Metolazone is chemically 7-chloro-2-methyl-3-(2-methylphenyl)-4-oxo-1, 2-dihydroquinazoline- 6-sulfonamide [3] (Structure-1). Metolazone is an oral diuretic drug, commonly classified with the thiazide diuretics. It is primarily used to treat congestive heart failure and high blood pressure. Metolazone indirectly decreases the amount of water reabsorbed into the bloodstream by the Kidney, so that blood volume decreases and urine volume increases. This lowers blood pressure and prevents excess fluid accumulation in heart failure. The emperícal formula for Metolazone is C16H16ClN3O3S and the molecular weight is 365.84 grams.
(Structur-1)
Literature review reveals that methods have been reported for analysis of Metolazone. Ultra-Violet and Derivative spectrophotometric methods for estimation of Metolazone in pharmaceuticals 2 &5-8.A Validated UV spectrophotometric method of Metolazone in Bulk and its Tablet Dosage forms 3&9-11. Validated RP-HPLC method for simultaneous quantitation of Losartan potassium and Metolazone in bulk drug and formulation4&12-17. Validated HPTLC Method for Simultaneous Estimation of Ramipril and Metolazone in Bulk Drug and formulation 1&18-21. LC-MS-MS development and validation for simultaneous quantitation of Metolazone with other drug in human plasma.
There is no published data on Validation of Metolazone by HPLC in bulk drug and in tablet dosage form by the use of mobile phase containing a mixture of Buffer, Methanol, and Acetonitrile in the proportion 65:28:7 respectively till to date. This present study reports there is no significant change in assay level observed up to 48 Hrs for test solution at room temperature. Thus, it can be concluded that the solution is stable up to 48Hrs at room temperature. The proposed method is validated as per ICH Guidelines.
There is however no reported HPLC and UV- Visible spectrophotometric method for the analysis of Metolazone in its technical grade and formulations.
This chapter describes a validated HPLC and UV- visible spectrophotometric methods for the quantitative determination of Metolozone. Functional group used for color development of Metolozone was primary amine group. The results obtained in this method was based on reaction of Metolozone with Oxidative coupling reaction with1, 10 – Phenanthroline. An attempt has been made to develop and validate all methods to ensure their accuracy, precision, repeatability, reproducibility and other analytical method validation parameters as mentioned in the various guidelines.
II. EXPERIMENTAL
A. Preparation of Standard Calibration Curve of Pure Drug
- Solvent
Dimethylsulfoxide was used as Solvent.
2. Preparation of Standard Stock Solution
Accurately weighed 100 mg of Metolazone was dissolved in 40 ml of Dimethylsulfoxide in 100 ml volumetric flask and volume was made up to the mark with Dimethylsulfoxide. i.e. 1000 µg ml-1 (Stock solution A)
From the above stock solution A 10 ml of solution was pipette out into 100 ml volumetric flask and the volume was made up to the mark with Dimethylsulfoxide to obtain the final concentration of 100 µg ml-1 (Stock solution B)
3. Preparation of Calibration Curve
Fresh aliquots of Metolazone ranging from 1 to 6 ml were transferred into a series of 10ml volumetric flasks to provide final concentration range of 10 to 60 µg/ml. To each flask 1ml of (0.01M) 1, 10-phenanthroline solution was added followed by 1ml of (0.2%) Ferric chloride solution and resulting solution was heated and finally 1ml (0.2M) Orthophosphoric acid solution was added. The solutions were cooled at room temperature and made up to mark with distilled water. The absorbance of orange red colored chromogen was measured at 520 nm against the reagent blank. The color species was stable for 24 h. The amount of Metolazone present in the sample solution was computed from its calibration curve.
4. Procedure for Formulations
Twenty tablets containing Metolazone were weighed and finely powdered. An accurately weighed portion of the powder equivalent to 100 mg of Metolazone was dissolved in a 100 ml of Dimethylsulfoxide and mixed for about 5 min and then filtered. The Dimethylsulfoxide was evaporated to dryness. The remaining portion of solution was diluted in a 100 ml volumetric flask to the volume with Dimethylsulfoxide up to 100 ml to get the stock solution A. 10ml of aliquots was pipette out into 100 ml volumetric flask and the volume was made up to the mark with Dimethylsulfoxide to obtained the final concentration of 100 µg ml-1 (Stock solution B).
Subsequent dilutions of this solution were made with Dimethylsulfoxide to get concentration of 10 to 60 µg ml-1 and were prepared as above and analyzed at the selected wavelength, 520 nm and the results were statistically validated
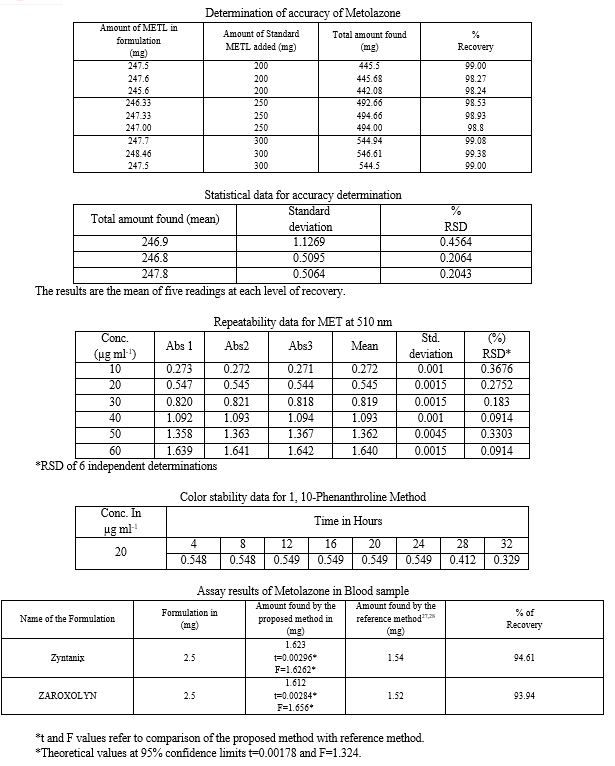
5. Procedure for Blood Sample
After collection of Blood sample it will be centrifuged. For isolation of Metolazone from plasma sample, Dimethylsulfoxide was used for protein precipitation. Liquid- Liquid extraction was performed with plasma by alkalinization with 1M NaOH, followed by extraction with 30% dichloromethane in Hexane. The upper organic layer was evaporated to dryness, and the dry residue was dissolved in 100 ml of Dimethylsulfoxide (1000 µg ml-1). From the above solution 10 ml is taken into a 100 ml of volumetric flask and made up to the mark with Dimethylsulfoxide. (100 µg ml-1)
From the above solution ranging from 1-6ml (10-60 µg ml-1) were transferred in to 10 ml volumetric flask and to the each flask 1ml of (0.01M) 1, 10- Phenanthroline solution was added followed by 1ml of (0.2%) Ferric chloride solution and made up to the mark with Dimethylsulfoxide. Then the resulting solution was heated and finally 1ml (0.2M) Orthophosphoric acid solution was added. The solutions were cooled at room temperature and made up to the mark with distilled water. The absorbance of orange red colored chromogen was measured at 520 nm against the reagent blank. The color species was stable for 24 h. The amount of Metolazone present in the sample solution was computed from its calibration curve.
III. RESULTS AND DISCUSSION
A. Optical Parameters
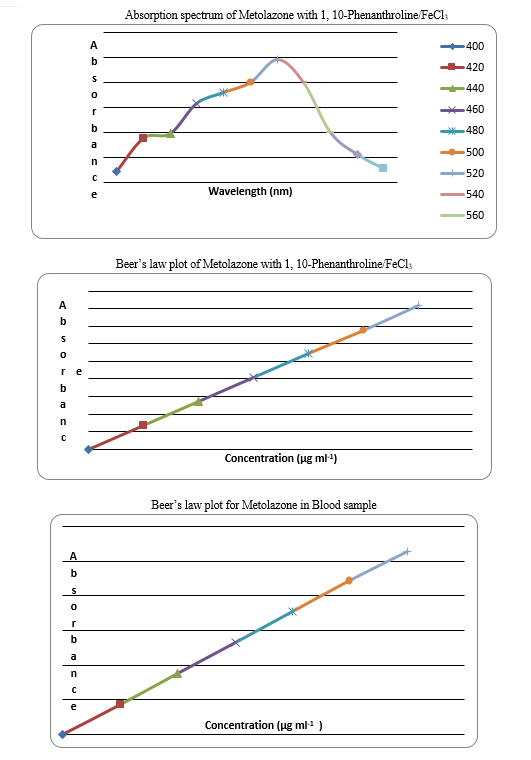
In order to ascertain the optimum wavelength of maximum absorption (λ max) formed in UV spectrophotometric method (Reference method – A) and of the colored species formed in each so the four visible spetrophotometric methods, specified amount of Metolazone in final solution 5 µg ml-1 (method A), 5 µg ml-1 (method B), were taken and the colors were developed following the above mentioned procedures individually.
The absorption spectra were scanned on spectrophotometer in the wavelength region of 200-400 nm (for method A) and 380-800 nm against corresponding reagent blanks. The regent blank absorption spectrum of each method was also recorded against distilled water /Dimethylsulfoxide. The results are graphically represented in (fig)
B. Parameters Fixation
In developing these methods, a systematic study of the effects of various relevant parameters in the methods concerned were under taken by verifying one parameter at a time and controlling all other parameter to get the maximum color development (for methods), reproducibility and reasonable period of stability of final colored species formed. The following studies were conducted.
The results obtained in this method were based on oxidation followed by complex formation reaction of Metolazone with 1,10-phenanthroline, Ferric chloride and Orthophosphoric acid to form an orange red colored chromogen that exhibited maximum absorption at 520 nm against the corresponding reagent blank. The functional group used for the color development for this method was primary amine group.
A schematic reaction mechanism of METL with 1, 10-Phenanthroline reagent was shown in (fig) the effect of various parameters such as concentration and volume of 1, 10- Phenanthroline and strength of acid order of addition of reagents, solvent for final dilution were studied by means of control experiments varying one parameters at a time.
C. Optical Characteristics
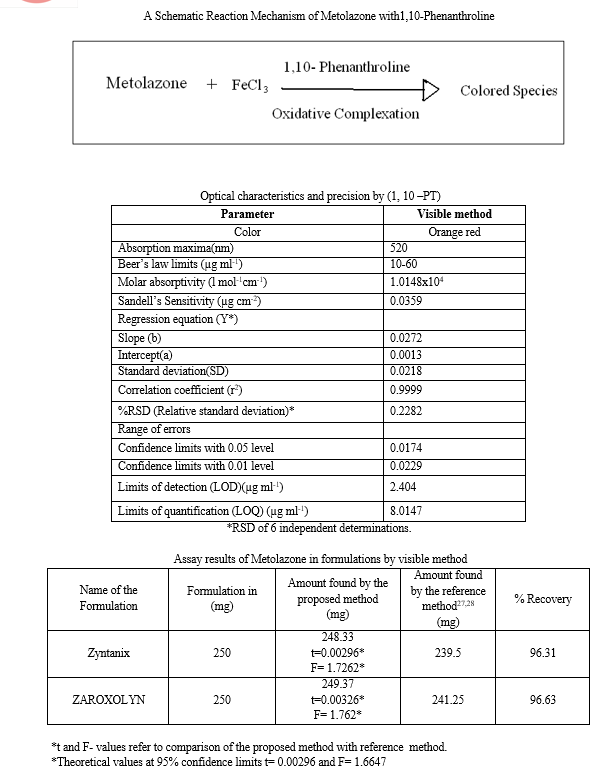
The reference method adhere to beer’s law the absorbance at appropriate wave length of a set of solutions contains different amounts of Metolazone and specified amount of reagents (as described in the recommended procedure) were noted against appropriate reagent blank. The beers law plot of the system illustrated graphically (fig: ) least square regression analysis was carried out for the slope. Intercept and Correlation Coefficient. Beer’s law limits, Molar absorptivity & Sandells sensitivity for Metolazone with each of mentioned reagents was calculated. The optical characteristics were present in the table.
In order to test whether the colored species formed in the methods B adhere the beer’s law the absorbance at appropriate wavelength of a set of solutions contain different amounts of Metolazone and specified amount of reagents ( as described in the recommended procedure) were noted against appropriate reagent blanks or distilled water. The beers law plots of the system illustrated graphically (fig:) least square regression analysis was carried out for the slope, intercept and correlation coefficient, beer’s law limits molar absorptivity Sandells sensitivity for Metolazone with each of mentioned reagents were calculated. The optical characteristics are presented in the Tables.
D. Precision
The precision of each one among the five proposed spectrophotometric methods were ascertained separately from the absorbance values obtained by actual determination of a fixed amount of Metolazone (5 & 5 µg/ml respectively – A&B) in final solution. The percent relative standard deviation and percent range of error (at 0.05 and 0.01 confidence limits) were calculated for the proposed methods and presented in Table.
E. Analysis of Formulations
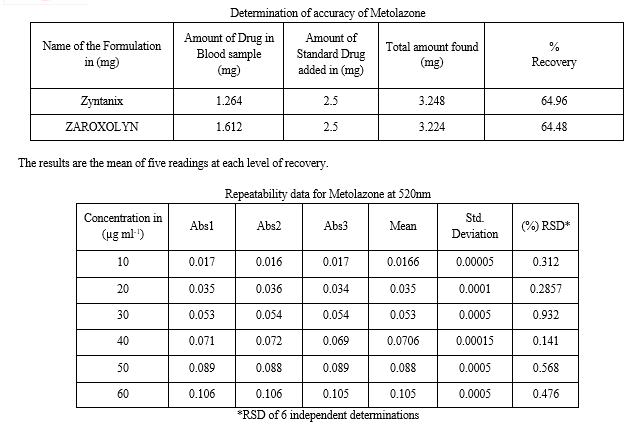
Commercial formulations of Metolazone were successfully analyzed by the proposed methods. The values obtained from the proposed and reference methods were compared statistically by the t and F tests and were found that those proposed methods do not differ significantly from the reported methods and they were presented in Tables. The proposed methods also applied for Biological Samples (Blood) for good recoveries are obtained which were recorded in Tables.
F. Accuracy
Recovery studies were carried by applying the method to Drugs sample present in formulations to which known amount of Metolazone of label claim was added (Standard addition method). The recovery studies were carried by applying the method to Biological sample (Blood) to which known amount of Metolazone correspond to 2mg Formulations taken by the patient. By the follow of Standard addition method 2 mg of label claim was added. After the addition of these standards the contents were transferred to 100ml volumetric flash and dissolved in solvent. Finally the volume was made up to the mark with solvent. The solution was filtered through Whitman No. 41filter paper. The mixed sample solutions were analyzed and their absorbance value was determined. At each level of recovery five determinations were performed and present in Tables. The results obtained were compared with expected results and were statistically validated in Tables.
G. Linearity and Range
The linearity of analytical method is its ability to elicit test results that are directly proportional to the concentration of analyze in sample with in a given range. The range of analytical method is the interval between the upper and lower levels of analyze that have been demonstrated within a suitable level of precision, accuracy and linearity.
H. Specificity and Selectivity
Specificity is a procedure to detect quantitatively the analyze in the presence of components that may be expected to the present in the sample matrix. While selectivity is a procedure to detect the analyze qualitatively in presence of components that may be expected to present in the sample matrix. The excipient in formulations was spiked in a pre weighed quantity of Drugs and then absorbance was measured and calculations were done to determine the quantity of the Drugs
I. Repeatability
Standard solutions of Metolazone were prepared and absorbance was measured against the solvent as the blank. The observance of the same concentration solution was measure five times and standard deviation was calculated and presented in Tables’
J. Interferences Studies
The effect of wide range of inactive, ingredients usually present in the formulations for the assay of Metolazone under optimum conditions was investigated. None of them interfered in the proposed methods even when they are present in excess fold than anticipated in formulations.
K. Solution Stability
The stability of the solutions under study was established by keeping the solution at room temperature for 48 Hours. The results indicate no significant change in assay values indicating stability of Drug in the solvent used during analysis. The results ar e recorded in Table .
References
[1] Jitendra A. Wayadande, Ramkumar: Validated HPTLC Method for Simultaneous Estimation of Ramipril and Metolazone in Bulk Drug and Formulation Der Pharmacia Sinica, 2011, 2 (4): 286-294 ,Pune Maharashtra, India [2] ShobhaManjunath,S.AppalaRaju:Ultraviolet and derivative spectrophotometric methods for estimation of Metolazone in pharmaceuticals.Vol-2,Issue-3, July-2011 ISSN: 0976-7908 Gulbarga – 585105, Karnataka. [3] B.DurgaPrasad1*,B.ChandraKanth2:AValidated UV Spectrophotometric method of Metolazone in Bulk and its Tablets Dosage forms. Durga Prasad B. et al. / International Journal of Biological & Pharmaceutical Research. 2012; 3(1): 154-157.ISSN 0976 – 3651: 2229-7480. [4] Ramkumar DUBEY, Vidhya K. BHUSARI : Validated RP-HPLC Method for Simultaneous Quantitation of Losartan Potassium and Metolazone in Bulk Drug and Formulation. This article is available from: http://dx.doi.org/10.3797/scipharm.1105-13 [5] Indian Pharmacopoeia, controller publication New Delhi 2007, 3, 1648 [6] United States Pharmacopoeia 32, Asian Edition NF27, The Official Compounds ofStandards 2009, 3, 3474. [7] United States Pharmacopoeia 32, Asian Edition NF27, The Official Compounds ofStandards 2009, 2, 2961. [8] Y. Gupta, A. Shrivastava, Asian Journal of Pharmaceutical and Clinical Research,2009, 2(4), 104-111. [9] L. Joseph, M. George and V. Rao, Pak. J. Pharm. Sci., 2008, 21(3), 282-284. [10] A. Sharma, B. Shah, B. Patel, Der Pharma Chemica, 2010, 2(4), 10-16. [11] K. Lakshmi, L. Sivasubramanian And K. Pal, International Journal of Pharmacy andPharmaceutical Sciences, 2010, 2(4), 126-129. [12] L. Potale, M. Damle, A. Khodke and K. Bothara, International Journal of PharmaceuticalSciences Review and Research, 2010, 2(2), 36-39. [13] A. Gaikwad , V.Rajurkar , T. Shivakumar ,G. Dama and H. Tare, Indo-Global Journal ofPharmaceutical Sciences, 2011,1(1), 99-112. [14] V. Jadhav, P. Mande, V. Kadam. International journal of pharmaceutical research anddevelopment, 2009, 2(5), 961. [15] M. Salvadori, F. Robert, B. Borges, H. Manistela; Cristina, RP Rolinson, A Moreno, N.Borges, Informa Healthcare-Clinical and experimental hyper tension, 2009, 31(5), 415. [16] S. Roy, K. Mangaonkar, S. Yetal, S. Joshi, E- Journal of Chemistry, 2007, 5(3), 634. [17] G Wei, S Xiao, C Liu, Journal of Chromatography B, 2006, 845(1), 169. [18] ICH Q2(R1) Validation of analytical procedures: text and methodology. Internationalconference on harmonization, Geneva, 2005. [19] V. Patel, P. Patel, B. Chaudhary, N. Rajgor, S. Rathi, International Journal on Pharmaceutical and Biological Research, 2010, 1(1), 18-24. [20] P. Mohite, R. Pandhare, V. Bhaskar, Eurasian J. Anal. Chem., 2010, 5(1), 89-94. [21] G. Bhavar, V. Chatpalliwar, D. Patil, and S. Surana, Indian J Pharm Sci., 2008, 70(4), 529–531. [22] A. Gaikwad , V.Rajurkar , T. Shivakumar ,G. Dama and H. Tare, Indo-Global Journal ofPharmaceutical Sciences, 2011,1(1), 99-112. [23] V. Jadhav, P. Mande, V. Kadam. International journal of pharmaceutical research anddevelopment, 2009, 2(5), 961. [24] M. Salvadori, F. Robert, B. Borges, H. Manistela; Cristina, RP Rolinson, A Moreno, N.Borges, Informa Healthcare-Clinical and experimental hyper tension, 2009, 31(5), 415. [25] S. Roy, K. Mangaonkar, S. Yetal, S. Joshi, E- Journal of Chemistry, 2007, 5(3), 634. [26] G Wei, S Xiao, C Liu, Journal of Chromatography B, 2006, 845(1), 169. ICH Q2(R1) Validation of analytical procedures: text and methodology. International conference on harmonization, Geneva, 2005. [27] Chatwal GR, Anand SKJ. Instrumental Methods of Chemical Analysis, Himalaya Publishing House, Mumbai, 2003:2.108-2.109 [28] Harris, D. C. (2003); “Quantitative Chemical Analysis 6th ed.”; 258-261, 407-422, first figure @ pp. 453, 461-476, 707-709. [29] Chilukuri S. P. Sastry, Kolli Rama Rao. Determination of Cefadroxil by three simple spectrophotometric methods using oxidative coupling reaction. Mkrochin Acta 126, 167-172 (2003) [30] Mohamed S. Mohamed and Etal, Metolazone co-crystals-loaded oral fast dissolving films: Design, optimization, and in vivo evaluation journal-of-drug-delivery-science-and-technology, Vol-90 December 2023, 105167 https://doi.org/10.1016/j.jddst.2023.105167 [31] Taylor D. Steuber, Kristin M. Janzen, Meredith L. Howard,A Systematic Review and Meta-Analysis of Metolazone Compared to Chlorothiazide for Treatment of Acute Decompensated Heart Failure, ACCP journal,2020 https://doi.org/10.1002/phar.2440 [32] Yub Raj Sedhai b, Suman Gaire a, Barun Babu Aryal a, Karan Singh b, Irfan Waheed b, Wasey Ali Yadullahi Mir c, Mohammad Saud Khan d, Jacquelene Dawson Dowe d, Mohammed Kazimuddin d, Soney Basnyat e, Ankush Asija f, Nimesh K. Patel g, Use of metolazone as an adjunct therapy to loop diuretics in diuretic resistant acute decompensation of heart failure: A systematic review and meta-analysis ELSEVIER,Vol -7June 2023, 100094 https://doi.org/10.1016/j.hsr.2023.100094 [33] Arati Chaudhary*, K.R. Vadalia and Punam Thummer, Chaudhary et al., DEVELOPMENT AND VALIDATION OF RATIO DERIVATIVE SPECTROPHOTOMETRIC FOR SIMULTANEOUS ESTIMATION OF METOLAZONE AND SPIRONOLACTONE IN PHARMACEUTICAL DOSAGE FORM, IJPSR, 2012; Vol. 3(10): 3999-4003 ISSN: 0975-8232 [34] Sunil R. DHANESHWAR and Etal , Simultaneous determination of metolazone and valsartan in plasma by on-line SPE coupled with liquid chromatography/tandem mass spectrometry, Sci. Pharm. 2011, 79(3), 545-554 Sci. Pharm. 2011, 79(3), 545-554; https://doi.org/10.3797/scipharm.1105-13 [35] F. E. Abeng and Etal, Metolazone compound as corrosion inhibitor for API 5L X-52 steel in hydrochloric acid solution , Bulletin of the Chemical Society of Ethiopia /,Vol. 34 No. 2 (2020) DOI:10.4314/bcse.v34i2.16 [36] Su Ern Yeoh Etal ,Dapagliflozin vs. metolazone in heart failure resistant to loop diuretics , European Heart Journal, Volume 44, Issue 31, 14 August 2023, Pages 2966–2977, https://doi.org/10.1093/eurheartj/ehad341 [37] Olga Zaporozhets, Iuna Tsyrulneva, Etal, Determination of 8 Diuretics and Probenecid in Human Urine by Gas Chromatography-Mass Spectrometry: Confirmation Procedure, American Journal of Analytical Chemistry Vol. 3 No. 4 (2012) , Article ID: 18437 , 8 pages DOI:10.4236/ajac.2012.34044 ******
Copyright
Copyright © 2024 Dr P. Suguna. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63157
Publish Date : 2024-06-06
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

