Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Versatile Platform of Novel Approaches in Transdermal Delivery
Authors: K. Shobana, S. Singarani, K. Sivaprakash, P. Yamini, S. Muhamed Satham Ussain
DOI Link: https://doi.org/10.22214/ijraset.2024.64063
Certificate: View Certificate
Abstract
Comprising of phospholipids, ethanol, and terpene, invasomes exhibit suitable transdermal penetration properties for soft vesicles. In comparison to liposomes and ethosomes, invasomes penetrate deeper into the skin. The ability to access the skin subcaste enhances the effectiveness of invasomes, which exert their effects by fluidizing the bilayer structure of stratum corneum (SC) lipids and disrupting lipid and intracellular protein interactions. They offer various advantages, including enhancing medication efficacy, improving patient compliance, and enhancing comfort. Over all the enhanced delivery of drugs through the skin and cellular membranes by means of an invasomal carrier opens numerous challenges and opportunities for research and future development of novel improved therapies.
Introduction
I. INTRODUCTION
Transdermal drug delivery is a clinically approved method for administering medicine through the skin. It offers a more comfortable route of administration and reduces the risk of fluctuations in blood drug concentrations and hazardous side effects. Invasomes are a type of artificial vesicle nanocarrier that transport substance through the skin, the body's most superficial biological barrier. These small particles, Invasomes are a form of liposomes that are flexible and consist of phospholipids, ethanol, and either one terpene or a combination of cells[1]. Ethanol improves the fluidity of lipids within the vesicles’ structure,
providing a soft and flexible shape that is less rigid than typical liposomes and, as a result, enhancing their skin permeability. It has been components.Invasomes are a type of artificial vesicle nanocarrier that transport substances through the skin, the body's most superficial biological barrier.These small particles, surrounded by a lipid layer,can carry substances into and out of cells. Invasomes are bilayer vesicles composed of soy phosphatidylcholine (SPC)lyso phosphatidylcholine (flexibility substances), terpenes, and ethanol (a permeation enhancer). The presence of penetrative boosters like terpene andethanol gives invasomes a high penetration potential.The incorporation of ethanol and terpenein invasomes facilitates lipid fluidity in the vesicle structure, making them more flexible and less rigid than typical liposomes. Ethanol interacts with lipids in the stratum corneum (SC) polar group region,causing structural changes in the keratinized andlipophilic regions, decreasing lipid transition temperatures, and fluidizing and disrupting the tightly packed SC lipids reported that terpenes increase penetration by disrupting the compound act structure of the SC lipids.surrounded by a lipid layer Transdermal, can carry substances into and out of cells[2] .
II. STRUCTURE OF INVASOME
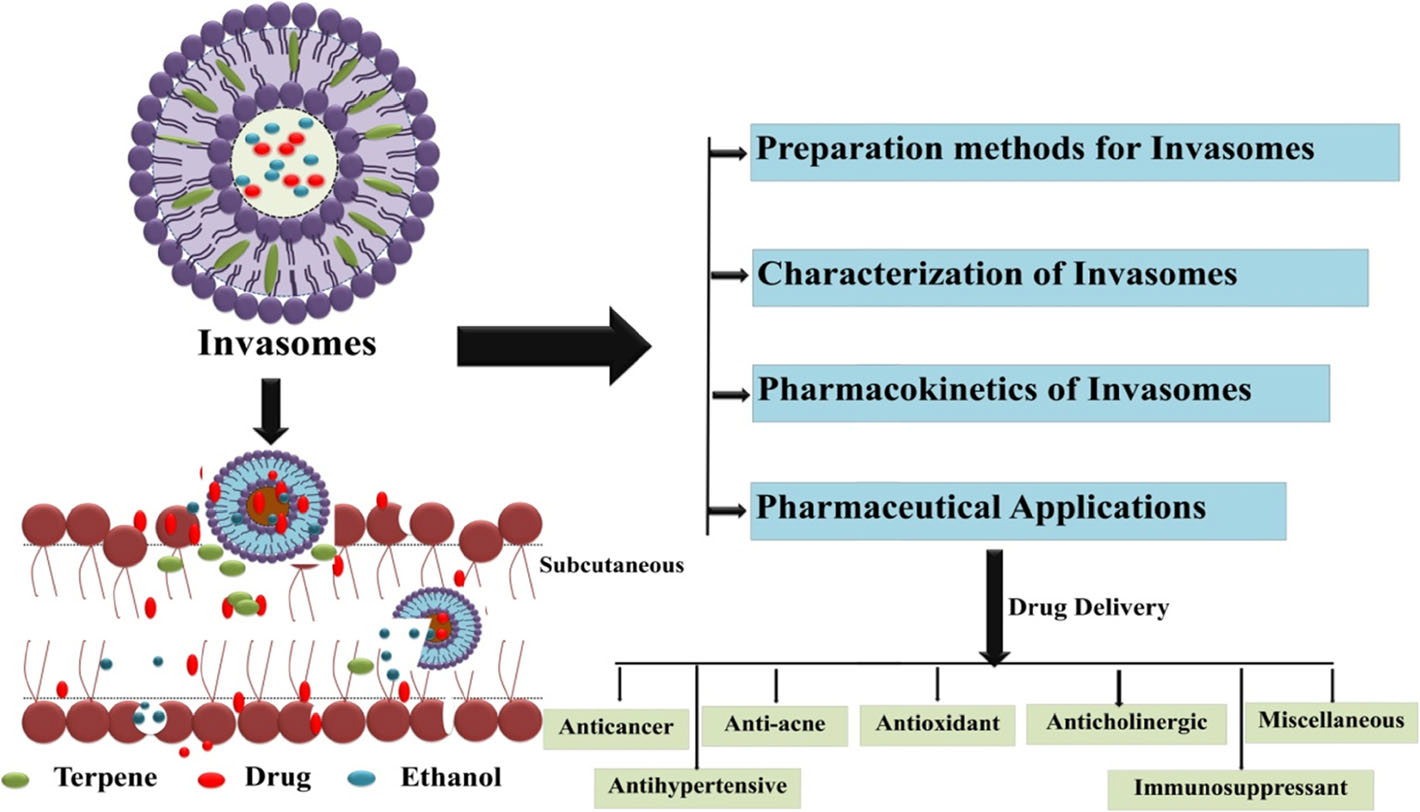
Figure 1: Invasome Structure and Applications
- Composition: The soft liposomal vesicles known as invasomes, which may operate as carriers with improved skin penetration, contain small quantities of ethanol and different terpenes or terpene combinations. They include phospholipids and a tiny amount of alcohol, as well as terpenoids (such as citral, limonene, and cineole), water, and a small quantity of ethanol (e.g., 3-3.3 percent by volume). Other components include terpenoids (such as citral, eugenol), water, and ethanol. To improve the absorption of hydrophilic and hydrophobic medications, terpenes (C5H8) have a general formula. The terpenes in essential oils are commonly used penetration enhancers. When used in low dosages, terpenes are less irritating to the skin. Terpenes are also considered safe by the FDA[3].
- Ethanol: Ethanol can be used to enhance permeability. Because of their particular size, zeta potential, entrapment efficacy, and skin permeability, vesicles in nano-vascular systems have a significant influence. According to various research, the vesicles’ size and entrapment effectiveness decrease as the ethanol concentration rises. The vesicles dissolve as the ethanol concentration rises. Increased ethanol levels reduce membrane thickness and consequently vesicular volume. Ether penetrates hydrocarbon chains and changes the net charge of vesicles, resulting in smaller average vesicles. Ethanol can also improve the fluidity of nanovesicles. Ethanol disrupts the densely packed structure of SC lipids, causing them to split. Because ethanol influences the structure of keratinize or lipophilic domains, it can lower the transition temperature of lipids. In comparison to liposomal nanovesicles, ethanol-based nanovesicles have a softer and less rigid structure. Because of their negative surface charge and electrostatic repulsion, ethanol nanovesicles may be more stable in storage.
- Terpenes: Terpenes or terpene mixtures in very low doses have also been proven to be penetration enhancers (also known as sorption boosters or accelerants) in transdermal drug delivery systems, allowing them to penetrate the skin and reduce barrier resistance. Terpenes pose little risk of skin irritation, hence they are classed as "Generally Recognised as Safe" (GRAS). Terpenes' capacity to permeate the skin is influenced by their solubility, the breakdown of lipid and protein layers, and the loss of skin micro-ingredients.
- Phospholipids: In phospholipids, water-loving hydrophobic acyl chains are connected to the alcohol. Distinctions in head groups, aliphatic chains, and alcohols allowed a diverse range of phospholipids to survive. As a result, the changed phospholipid sources benefit the phospholipid classes. Natural and synthetic phospholipids, such as PEGylated Phospholipids, are used in a variety of formulations, including those for skin care products.[4,5]
A. Advantages
- It is a non-invasive drug delivery technique.
- Better patient compliance.
- It is more stable than other ultra-deformable vesicles.
- The formulation contains no toxic raw materials.
- Targeted drug delivery of hydrophilic and lipophilic drugs is possible.
- It can easily penetrate through skin layers.
- Compared to iontophoresis or phonophoresis and with other complicated techniques, this is simple method for the delivery of the drugs.
- Patient compliance is better as the drug can be administered as semisolid form [6]
B. Disadvantages
- It requires high cost for production
- Chance of leakage and fusion of encapsulated active.
- Invasome containing phospholipids may get oxidized/hydrolyzed and affect the stability of vesicles.[6]
C. Applications
- Anastrazole invasomal gel is used for breast cancer therapy in post- menopausal women.
- Dapsone loaded invasome is used in acne therapy. dermatological conditions.
- Finasteride and Minoxidil loaded invasome is used for the treatment of Alopecia.
- Skin penetration and bioavailability of Avanafil for the treatment of Erectile Dysfunction is improved via invasomal formulation.
- Isotretinoin invasomal preparation is a vitamin A analogue used to treat Eosinophilic Pustular Folliculitis.[7]
III. EVALUATION OF INVASOMES
- Penetration Enhancement
- Flexibility
- High Encapsulation Efficiency
- Stability
- Controlled and Targeted Drug Release Entrapment Efficiency
- Surface Morphology
- Drug Content
- Vesicular size
IV. INVASOME PENETRATION MECHANISM
Terpenes and Ethanol Invasomes act as osmotic agents by promoting the deformability of vesicles, damaging the bilayer skeleton of SC, and increasing the permeability of invasomes. Some of the vesicles disintegrate, claiming to release their components such as terpenes, phospholipid segments. Non-collapsed invasive vesicles pass through the SC. Invasome can reach the internal regions of SC via follicular transport pathways. When the invasome reaches SC, many of them collapse, but smaller vesicles and flexible invasomes pass through deeper layers.[8]
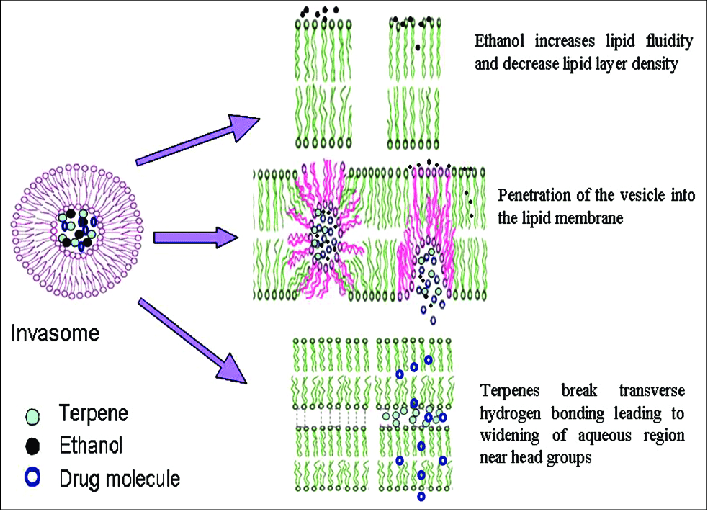
Figure 2: Mechanism of invasome penetration
V. METHODS OF PREPARATION OF INVASOME
A. Mechanical Dispersion Method
In an ethanolic phospholipid solution, a drug and a terpene or terpene combination are dissolved. To create a clear solution, the mixture is vortexed for 5 minutes and then sonicated for 5 minutes. A syringe is used to add phosphate buffer saline (PBS) (pH: 7.4) to the solution, which is constantly vortexed. The vortexing is maintained for another 5 minutes to achieve the final invasomal preparation.
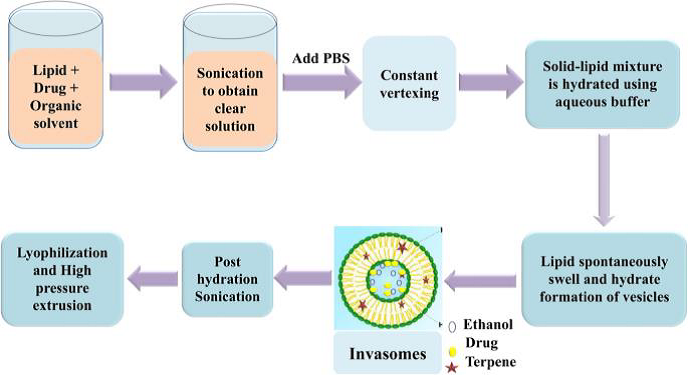
Figure 3: Mechanical dispersion method
B. Thin film Hydration Method
The traditional film process can also be used to prepare invasomes. Phospholipids in ethanol are dissolved in a 2:1 v/v mixture of methanol and chloroform. The rotary flash evaporator is used to dry this mixture to a thin film by gradually lowering the pressure from 500 to 1 mbar at 50°C. The film is vacuumed (1 mbar) for 2 hours at room temperature before being flushed with nitrogen. The film is then hydrated for 30 minutes at the lipid phase transition with a mixture of phosphate buffer (pH: 7.4; PBS) containing ethanol and terpenes, or it is hydrated with PBS (pH: 7.4) and after cooling to room temperature,ethanol and a single terpene or a terpene mixture is added to obtain invasomes.[9
Figure 4 : Thin film hydration Method
C. Storage Conditions
Invasomes should be stored in a cool and dry environment to prevent degradation. They are often refrigerated at temperatures between 2 to 8°C. It's important to protect them from light and moisture, as these factors can affect their stability. Invasomes should be stored in airtight containers which helps to minimize exposure to oxygen body’s systemic circulation via patches.[10]
VI. CHARACTERISATION OF INVASOME
- Entrapment Efficiency
- Surface Morphology
- Drug Content
- Vesicular size
- Ex Vivo Permeation Studies
- Stability Studies
- Entrapment Efficiency: The trapping efficiency was investigated utilizing ultracentrifugation. For this, 0.1 ml of the invasomal preparation was transferred to Eppendorf tubes and centrifuged at 15,000 rpm at 4°C for 15 minutes, repeated for two cycles to isolate untrapped drugs. The transparent portion obtained after centrifugation was utilized to determine the concentration of free drug. The percentage of trapped drug was indirectly calculated based on the amount of free drug using a specific formula. This method allows for the quantification and assessment of the efficiency of drug entrapment within the invasomal vesicles, providing valuable insights for drug delivery and formulation optimization. Entrapment Efficiency (%) = total drug −free total drug × 100
- Surface Morphology: The evaluation was conducted by applying a drop of the preparation onto a transparent, air-dried glass slide coated with gold using a sputter coater and subsequently visualizing it under a scanning electron microscope. The samples were then tightly sealed in 10 ml glass vials and stored under refrigeration (4 - 8°C) and at room temperature for one month. Throughout this period, the trapping efficiency and visual appearance were regularly assessed. This method ensures the stability and integrity of the formulation over time, providing crucial insights into its long-term storage characteristics and potential applications in various fields.
- Drug Content: The drug content within invasomes can be assessed using an ultraviolet spectrophotometer, which enables precise quantification. Additionally, an enhanced high-performance liquid chromatography (HPLC) method allows for more accurate and sensitive determination of the drug content. These analytical techniques provide valuable tools for researchers and pharmaceutical scientists to ensure the consistency and quality of invasome formulations, facilitating their development and optimization for various therapeutic applications.[9]
- Vesicular Size and Shape: Invasomes can be observed using both transmission electron microscopy (TEM) and scanning electron microscopy (SEM) techniques, offering insights into their structural characteristics and morphology. Additionally, the vesicle size and potential zeta particle size of invaginations within invasomes can be precisely determined through dynamic light scattering (DLS) and photon correlation spectroscopy methods. These analytical approaches provide comprehensive data on the size distribution and surface charge of invasomes, aiding in the optimization of their formulation and understanding their behavior in various applications, including drug delivery and biomedical research.
- Ex-vivo Permeation Studies: The osmolarity of the invasome formulations was assessed using a Franz diffusion cell setup. The diffusion cell, with an effective surface area of 2.0 cm² and a receptor volume of 20 ml, was utilized for the experiment. The skin was mounted onto the receiving compartment, with the stratum corneum side facing upwards, while the donor compartment was filled with the invasome preparation. A cap covered the top of the diffusion cell to maintain the experimental conditions. Phosphate-buffered saline at pH 7.4, kept at 37°C, served as the recipient medium with a volume of 20 ml. Throughout the experiment, aliquots were periodically withdrawn and replaced with fresh medium to ensure optimal conditions. The samples collected were analyzed using a UV spectrophotometer to quantify the osmolarity of the invasome formulations accurately.[11]
- In-Vitro Drug Diffusion Study: The in-vitro diffusion study is carried by using franz diffusion cell. Egg membrane is taken as semi permeable membrane for diffusion. egg membrane is mounted between the donor and the receptor compartment. A two cm2 size patch taken and weighed then placed on one side of membrane facing donor compartment. The receptor medium is phosphate buffer pH 7.4. The receptor compartment is surrounded by water jacket so as to maintain the temperature at 32±0.5°C. Heat is provided using a thermostatic hot plate with a magnetic stirrer. The receptor fluid is stirred by Teflon coated magnetic bead which is placed in the diffusion cell[12].
- Stability Studies: The physical stability of the optimized formulation was assessed to investigate drug leaching from the vesicles. The invasome gel samples were sealed in two 10 ml ointment tubes, with one tube stored in the refrigerator at 4°C to 8°C and the second tube maintained at 27°C-30°C, representing environmental temperature conditions. Over the course of one month, the samples were monitored weekly to evaluate any changes in form, drug content, and viscosity. This systematic evaluation helps ascertain the formulation's robustness and suitability for storage under different temperature conditions, providing insights into its shelf-life and stability profile.[13]
VII. PHARMACEUTICAL APPLICATION OF INVASOMES
- Delivery of Antiacne Agent: Dapsone-loaded invasomes using the film hydration technique, incorporating terpenes (such as limonene, cineole, citral, or fenchone) and phosphatidylcholine for treating mild to moderate acne.
- Anticancer Drug Delivery: Temoporfin, a potent second-generation photosensitizer, shows promising attributes such as high tumor selectivity and residual photosensitivity within just 2 weeks. Consequently, it holds potential as an effective anticancer agent in photodynamic therapy for early or recurrent cancer. In this regard, Dragicevic-Curic et al. reported the deposition of a highly hydrophobic photosensitizer, temoporfin, within the skin layer (SC) using invasomes[14].
- Immunosuppressive Drug Delivery: Nanotechnology holds potential as an alternative administration route for immunosuppressants. light of lipid vesicle substructures in drug delivery, the development of advanced nanosized vesicles emerges as a crucial focus for researchers.
- Delivery of Antihypertensive Agent: Antihypertensive drugs play a critical role in managing high blood pressure, yet they often face challenges related to low water solubility, poor bioavailability, short biological half-life, low permeability, and a list of undesirable side effects.
- Antioxidant: Ferulic acid invasomes exhibited superior permeability due to their deformable vesicles and the interaction of penetration enhancers with lipid lamellae and skin layers.This study underscores the potential of invasomes as effective carriers for enhancing the transdermal delivery of ferulic acid, offering promising implications for various therapeutic applications[15]
Conclusion
The invasomes, which have the potential to be a useful tool for medication delivery via the skin and have superior skin permeability. Invasomes have been tested to encapsulate hydrophilic drugs and hydrophobic drugs. Hence, they can open up new challenges and opportunities for the development of novel improved therapies. The mechanical dispersion and film hydration techniques are effective methods for preparing invasomes, ensuring optimal encapsulation and stability.
References
[1] Raslamol K*1, AiswaryaI S2, Dilshana A S3, Mohamed Shahil4, Mumthas NA5, Sandra MS6 1D Invasome: A New Sight in Transdermal drugdelivery System. International Journal of Pharmaceutical Research and Applications Volume 9, Issue 3 May-June 2024, pp: 1093-1098 www.ijprajournal.com ISSN: 2456-4494 (1093-94) [2] Vidya, K., & Lakshmi, P. K. (2019). Cytotoxic effect of transdermal invasomal anastrozole gel on MCF-7 breast cancer cell line. Journal of Applied Pharmaceutical Science, 9(3), 50-58. [3] Dragicevic-Curic, N., Scheglmann, D., Albrecht, V., & Fahr, A. (2008). Temoporfin-loaded invasomes: development, characterization and in vitro skin penetration studies. Journal of Controlled Release, 127(1), 59-69. [4] Harshita Verma1*, Dr. Pradeep Pal2 Formulation, Development and Evaluation of Invasomes Loaded Clotrimazole Gel for Fungal Treatment Scholars Academic Journal of Pharmacy Abbreviated Key Title: Sch Acad J Pharm ISSN 2347-9531 (Print) | ISSN 2320-4206 [5] Dermatologic Harding CR. “The stratum corneum: structure and function in health and disease”. therapy 17 (2004): 6-15 [6] Alkilani AZ, McCrudden MT and Donnelly RF. “Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum”. Pharmaceutics 7.4 (2015): 438-470 [7] Dragicevic N, Verma DD and Fahr A. “Invasomes: Vesicles for enhanced skin delivery of drugs. In Percutaneous penetration enhancers chemical methods in penetration enhancement”. Springer, Berlin, Heidelberg (2016): 77-92. [8] Jain S, Tripathi S and Tripathi PK. “Invasomes: Potential vesicular systems for transdermal delivery of drug molecules”. Journal of Drug Delivery Science and Technology (2021): 102166. [9] D Singh. “Ultra deformable system: a carrier for transdermal drug delivery”. IJPSR 4 (2013): 4098-4104. [10] S Babaie., et al. “Invasome: A novel nanocarrier for transdermal drug delivery”. Nanomaterials 10.2 (2020): 341 [11] ND Curic, D Scheglmann, V Albrecht and A Fahr. “Temoporfin-loaded invasomes: Development, characterization and in vitro skin penetration studies”. J. Control. Release 127 (2008): 59-69. [12] L Jing., et al. “A review on phospholipids and their main applications in drug delivery systems”. Asian J. Pharm. Sci 10 (2015): 8198. [13] PK Lakshmi, V Mounica, M Kumar and D Prasanthi. “Preparation and evaluation of curcumin invasomes”. Int. J. Drug Deliv 6 (2014): 113-120. [14] Omar S Salih* and Entidhar J Al-akkam Invasomes as a Novel Delivery Carrier for Transdermal Delivery: Review Article Medicon Pharmaceutical Sciences Volume 2 Issue 3 April 2022. [15] Shikha Jain, Vikas Jain, and SC Mahajan, \'Lipid Based Vesicular Drug Delivery Systems\', Advances in Pharmaceutics, 2014 (2014), 1-12.
Copyright
Copyright © 2024 K. Shobana, S. Singarani, K. Sivaprakash, P. Yamini, S. Muhamed Satham Ussain. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64063
Publish Date : 2024-08-23
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

