Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Review on “Chromatography: A Versatile Tool in Pharmaceutical Analysis and Allied Industries
Authors: Rohit Pradip Ade , Rutuja Vinayak Bhavar , Sahil Namdev Darade , Anagha Amit Sarvadnya
DOI Link: https://doi.org/10.22214/ijraset.2025.66266
Certificate: View Certificate
Abstract
In pharmaceutical analysis, chromatography is a crucial analytical method that is extensively utilized to separate, determine, and measure the components of complicated mixtures. An outline of the basic ideas, historical evolution, and diverse uses of chromatography in the pharmaceutical and related sectors is given in this review. Chromatography, which began with Tswett\'s groundbreaking discovery in 1906, has developed into a flexible technique that uses both stationary and mobile phases for separation. The mechanics, guiding principles, and unique uses of techniques such as Gas Chromatography (GC), Liquid Chromatography (LC), (TLC), (HPLC), and High-Performance Thin Layer Chromatography (HPTLC) are thoroughly examined. The review explores the importance of immiscible phases in accomplishing selective molecule separation as well as partition and adsorption as the main separation methods. Applications cover a extensive range of domains, including as food safety, forensic science, environmental monitoring, quality control, and drug development. Chromatographic techniques have become essential for evaluating the purity of pharmaceutical products, guaranteeing their safety, and complying with regulatory requirements thanks to the integration of technological breakthroughs. The diversity of chromatography and its vital function in analytical research and industry are highlighted by this thorough study.
Introduction
I. ANALYTICAL TECHNIQUES
Pharmaceutical analysis uses analytical techniques to determine and measure the constituents of pharmaceutical product.
These techniques ensure that medications are safe, effective, and high quality. Here is a brief rundown of several fundamental methods:
II. INTRODUCTION
Chromatography: A widely utilized method for classifying and identifying species in biological, inorganic, and organic materials. utilized to separate component mixtures. elimination of contaminants.
The Russian scientist Tswett (1906) is credited with coining the term chromatography. He described the process of extracting various coloured elements from leaves using an appropriate solvent and then running the extract through a column consisting of sucrose, calcium carbonate, and alumina. The Greek term "chromon," which means "colour," is where the word "chromatography" originated. Chromatography is defined by the International Union of Pure and Applied Chemistry (I.U.P.A.C.) as a physical technique for separating mixture components. The components that need to be separated are split between the stationary phase and the mobile phase, according to I.U.P.A.C. A stationary phase can take many different forms, although it is often a column. A suitable liquid serves as the mobile phase .
These days, chromatography is a very versatile technique;
GC, LC, and TLC are the methods that chromatography can use to separate gases and volatile compounds; LC can separate involatile chemicals and materials with very high molecular weight, including biopolymers; and TLC can separate things very cheaply if needed. Because of their shared characteristics, all three methods—GC, LC, and TLC—are categorized as chromatography systems.
III. DEFINITION
chromatography, which is more precisely described as a physical technique of separation that allows a mixture of substances to be separated, isolated, and purified into distinct molecules that have varying rates of distribution based on 1. Solubility 2. Affinity (whether the molecules are polar or not) 3. Interaction with fixed material (the stationary phase, which we shall define later): The mixture's constituent parts are distributed in the space between the mobile and stationary phase, which travels in a predetermined direction at different speeds. The definition of chromatography is as follows: chromatography is the procedure of separating elements that make up a combination by dividing them between two stages, mobile and stationary phase.
Any chromatographic separation method needs to have these three essential components: 1. The Mobile phase 2. The Sample 3. The stationary condition .
IV. PRINCIPLE
Two immiscible drugs are utilized to break down a combination into its constituent parts. These are the stationary phase (which could be either a liquid or a solid) that adsorbs the mixture's components and the mobile phase (which could be either a liquid or a gas) that transfers the mixture's components to it as it passes through the stationary phase. The mixture's constituent parts are moved at various speeds. Along with the moving phase, the weakly adsorbed component moves faster and the strongly adsorbed component moves more slowly. Consequently, as the mobile phase passes through the stationary phase differentially adsorbed components are separated.
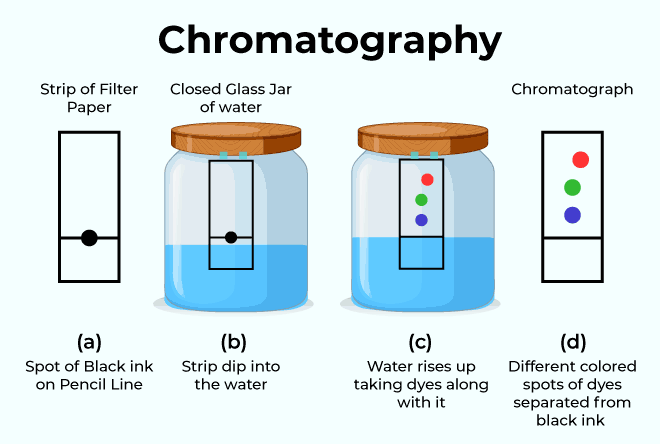
Fig.1: Basic figure of chromatography
Table.1: Types of Chromatography
|
Types of chromatography |
Stationary phase |
Mobile phase |
Principle |
|
Paper chromatography |
Liquid |
Liquid |
Partition |
|
Thin layer chromatography (TLC) |
Solid |
Liquid |
Partition |
|
Column chromatography |
Solid |
Liquid |
Adsorption |
|
Gas chromatography (GC) |
Solid or liquid |
Gas |
Separation |
|
High performance liquid chromatography (HPLC) |
Liquid |
Liquid |
Separation |
|
High performance thin layer chromatography (HPTLC) |
Liquid |
Liquid |
Separation |
V. TYPESCHROMATOGRAPHY
Chromatography can be of different type depending on the process of either adsorption or partition.
This method divides a mixture of substances into a moving solvent (movable phase) and a stagnant liquid phase (held on an appropriate solid support). Liquid-liquid chromatography is the term for chromatography in which the moving phase is a liquid. Gas-liquid chromatography is the term for chromatography in which the moving phase is a gas. Using cellulose or moist silica gel, liquid-liquid chromatography is the previous method of chromatography. Paper chromatography and thin layer chromatography are two examples. If the adsorbent is arranged into columns, the process is referred to as column chromatography. In each of these chromatographic methods, the medium serves as a water support.
Different types of chromatography are summarised in above Table 1.
A. Paper Chromatography
One kind of partition chromatography is paper chromatography in which two liquid are used and the components are distributed between them.one liquid is stationary phase (mostly water) that remain in paper, and the other liquid is the movable phase (development solvent). In partition chromatography, the water that is polarly adsorbed on the paper functions as the phase that is stationary for the 2D plate, and the movable and stagnant phases are both liquids. After the sample has been lysed, place it in a little spot 0.5 inches from the filter paper's edge and let it dry. The end nearest to the sample is in touch with the solvent and moves up and down by capillary action, holding the dry spot in the front end of an air-saturated closed chamber (whether the ascending agent rises depends on the method of action). It is distinct. Because of the phase's increased viscosity, it travels down or along the paper.
B. Thin Layer Chromatography
Is based on the principal of adsorption chromatography or partition chromatography. Used for qualitative and quantitative determination. Non-volatile mixtures can be separated using a process called thin layer chromatography. It is carried out on a TLC plate composed of a thin layer of adsorbent material covering a non-reactive solid. The material usually used is aluminium oxide, cellulose, or silica gel. Is founded on the principle of partition chromatography.
 Fig.2: Application of TLC
Fig.2: Application of TLC
The technique was developed in 20th century and it is still getting evolved with the advancement of technology.
In thin-layer chromatography, the movable phase is liquid, and the stagnant phase is a solid. These phases interact with a vast surface area to generate a solid liquid adsorption. The mobile phase (thin plate submerged in solution) is forced using the stagnant phase by capillary action. Polarization of the material, the solid phase, and the solvent all influence this upward migration rate. In order to distinguish each faint platelet colour that does not show up on the chromatogram as a distinct peak, coloured chemicals can be employed.
1) Principle of TLC
The basis of TLC is founded on the variations in the capillary action and adsorption of various compounds contained in complicated mixtures.
This method applies the sample to a thin coating of (silica gel or alumina) for the stagnant phase, which is then bonded to a flat, inert support such as an aluminium plate, glass, or plastic. The loaded sample-filled stationary phase is thereafter submerged in an appropriate solvent called as the movable phase. Capillary action causes the liquid to rise in the thin stationary phase plate.
C. Column Chromatography
The column can have an open or packed tubular geometry, according to an outline of a three-dimensional shape model The stationary phase fills the wall and all of the columns accessible space when it packed. However, the open tubulars stationary phase is situated at the column sites. When the mixture that has to be divided and the movable phase are injected from the columns top , the constituent elements of the mixture travel at different speeds.
 Fig 3:Application of column chromatography
Fig 3:Application of column chromatography
D. Gas Chromatography
This method of separation uses solid or liquid as the stagnant phase and a gas as mobile phase. If the stagnant phase is solid it is known as gas solid chromatography. Sample components are divided on the based on partition chromatography.The gas used in gas chromatography must be non-reactive or inert. The parts are divided as vapours in gas chromatography, which sets it apart from other types of chromatography. As a result, it is employed in the gas phase to identify and segregate tiny weight of molecules. The sample is vaporized in the injection port and may be a gas or a liquid. Because helium is chemically inert and has a low molecular weight, it is commonly used as the movable phase in gas chromatography. The analyte is transfer through the column by the movable phase when pressure is applied.
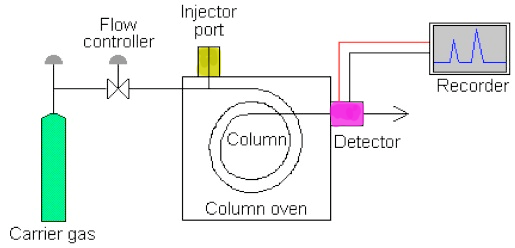
Fig.4: Block diagram of a gas chromatography system
E. High-Performance Liquid Chromatography (HPLC)
The analytical method known as HPLC, is used to distinct, identify, or quantify each component in a mixture. Mixtures are recognized and quantified spectroscopically after being separated according to the fundamentals of column chromatography.
His HPLC, which used metal columns suitable for high pressures, developed from LC column chromatography in the 1960s, which used glass columns suitable for low pressures.
(HPLC), which is used to divided and analyse the constituents of liquid samples.
1) Principle of HPLC
The purification area between the fixed and mobile phases is a separate column with a stationary phase consisting of a particulate matter composed of tiny porous particles additionally , a mobile phase made up of at a solvent or solvent mixture is run at under extreme pressure through a separation column . The specimen is injecting using a syringe into the movable phase flow that passes between the separation column and the pump through a valve that is attached to the sample loop, which is a capillary or stainless-steel tub.
 Fig.5: High Performance Liquid Chromatography Instrumentation
Fig.5: High Performance Liquid Chromatography Instrumentation
F. High-Performance Thin Layer Chromatography (HPTLC)
HPTLC is an improved TLC approach that makes better use of the traditional TLC process. Since the phase that is stationary is employed as a flatbed-like surface, it is sometimes referred to as planar chromatography or flat-bed chromatography.
1) Principle of HPTLC
HPTLC operates on the same adsorption-based separation mechanism as traditional TLC. The capillary action is traversed by the movable phase. The affinities of the analytes for the (adsorbent) in the stationary phase determine how they migrate. Components that have a more affirmation for the stagnant phase move more slowly, whereas those that have a less affinity move more quickly.
VI. APPLICATION OF CHROMATOGRAPHY
- In Pharmaceutical Applications: Assess duration of shelf life of pharmaceutical products. Determine which dosage forms' active ingredients are. Create an environment for pharmaceuticals. It is employed in the quality and purity testing of drugs.
- Material Sciences: This field is used to characterize materials, polymers, and nanoparticles. Inspection of quality pharmaceutical applications assess the shelf-life of pharmaceutical products.
- Life Sciences: It is employed in the process of sorting , recognizing , and measuring individual molecules from living samples.
- Quality control: Regarding applications in the environment Find diphenhydramine in samples that have been deposited. environmental biomonitoring.
- In the Medical Setting: Finding endogenous neuropeptides in extracellular fluids of the brai
- Food Industry: It is employed in the food quality testing and contamination detection processes.
- In the pharmaceutical industry: When identifying the existence of compounds or trace elements. This is also working in the division of compounds based on elemental composition and weight of molecules. in the purity of mixes and in the recognition of unfamiliar compounds. during the drug's development.
- In the chemical sector, polychlorinated biphenyls (PCBs) in oils and pesticides are among the many pollutants that are found using HPLC and GC.
- In the Food industry: In the recognition of additives and food spoilage. while analysing the nutrients in food both quantitatively and qualitatively.
- In Forensic Science: Hair and blood samples from crime scenes are analysed using this approach in forensic science.
- Environment Science: It is employed for recognition and analysis of pollutants .
Conclusion
Chromatography is a basic analytical method that is utilized extensively in many different domains, such as environmental sciences, life sciences, material sciences, and medicines. It allows for the possibility of to divide, detected, and measure the constituents of intricate mixes. Analyte differential partitioning between a mobile and stagnant phase is the technique\'s foundation. Specific analytical demands are met by a variety of chromatography techniques, including Gas Chromatography (GC), Paper Chromatography, (TLC), Column Chromatography, (HPLC), and (HPTLC). Chromatography is versatile because it can handle a variety of samples, including liquids, solids, and gases, while maintaining accurate and effective separation. Its uses range from environmental monitoring and medicine quality control to food safety analysis and forensic investigations. Chromatographic techniques\' constant development improves their sensitivity, effectiveness, and adaptability, guaranteeing their crucial place in the analytical sciences for many years to come.
References
[1] Dr. Vishnu. P. Chaudhari, Dr. Arti. Swami \'Pharmaceutical Analysis\' by Tec Knowledge publication, page no. 1.1 to 1.7 [2] https://www.ssi.shimadzu.com/service-support/faq/gas-chromatography/what-is-gas-chromatography/index.html [3] David G. Watson, Pharmaceutical Analysis, International 5th edition, 2017 [4] Gurudeep R. Chatwal, Sham K. Anand “Instrumental Methods of Analysis” By Himalaya Publishing House. [5] Dr. R. Sundhararajan, Dr. M.V. Kumudhavalli, Dr. Minal. T. Harde “Quality Assurance” By Thakur Publication PVT. LTD. Lucknow page no – 11-13. [6] Cuatrecasas P, Wilchek M, Anfinsen CB. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A 1968; 61:636–43. [7] Porath J. From gel filtration to adsorptive size exclusion. J Protein Chem 1997; 16:463–8. [8] Harris DC. Exploring chemical analysis. 3rd WH. Freeman&Co 2004. [9] http:/80.251.40.59/veterinary.ankara.edu.tr/fidanci/Ders_Notlari/Biyoteknoloji/Kromatografi.html. [10] Regnier FE. High-performance liquid chromatography of biopolymers. Science 1983. p. 245. [11] Stoddard JM, Nguyen L, Mata-Chavez H, Nguyen K. TLC plates as a convenient platform for solvent-free reactions. Chem Commun (Camb) 2007; 12:1240–1. [12] Sherman J, Fried B, Dekker M. Handbook of Thin-Layer Chromatography New York, NY; 199. [13] Donald PL, Lampman GM, Kritz GS, Randall G. Engel Introduction to Organic Laboratory Techniques (4th Ed.). Thomson Brooks/Cole 2006. p. 797–817. [14] https://dept.harpercollege.edu/chemistry/chm/100/dgodambe/thedisk/chrom/wback3.htm [15] https://chem.libretexts.org/Ancillary_Materials/Demos_Techniques_and_Experiments/General_Lab_Techniques/Thin_Layer_Chromatography [16] https://www.chemguide.co.uk/analysis/chromatography/thinlayer.html [17] https://pubmed.ncbi.nlm.nih.gov/24182936/ [18] A Review of Chromatograph: Principal, Classification, Application Miss. Vandana Samadhan Dongare, Dr. Nitin B. Kohale, P Mr. Suraj B. Rathod International Journal of Humanities Social Science and Management (IJHSSM) Volume 3, Issue 2, Mar.-Apr. 2023, pp: 367-373. [19] Bergh, J.J. and Breytenbach, J.C., 1987. Stability-indicating high-performance liquid chromatographic analysis of trimethoprim in pharmaceuticals. Journal of Chromatography A, 387, pp.528-531. [25]. Stubbs, C. and Kanfer, I., 1990. [20] Bounine, J.P., Tardif, B., Beltran, P. and Mazzo, D.J., 1994. High-performance liquid chromatographic stability-indicating determination of zopiclone in tablets. Journal of Chromatography A, 677(1), pp.87-93. [21] DE ZEEUW, R.A., 1969. Paper and thin layer chromatographic techniques for separation and identification of barbiturates and related hypnotics. In Progress in Chemical Toxicology (Vol. 4, pp. 59-142). Elsevier. [12]. Chittum, J. W. (1957). [22] © The Author(s) 2023 V. K. Ahluwalia, Instrumental Methods of Chemical Analysis, https://doi.org10.1007/978-3-031-38355-7_2 [23] A Review: Uses of Gas Chromatography-Mass Spectrometry (GC-MS) Technique for Analysis of Bioactive Natural Compounds of Some Plants Abeer Fauzi Al-Rubaye1, Imad Hadi Hameed2*, Mohanad Jawad Kadhim3 International Journal of Toxicological and Pharmacological Research 2017; 9(1); 81-85. [24] Cuatrecasas P, Wilchek M, Anfinsen CB. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A 1968; 61:636–43. [25] Porath J. From gel filtration to adsorptive size exclusion. J Protein Chem 1997; 16:463–8. [26] Fredj, G., Paillet, M., Aussel, F., Brouard, A., Barreteau, H., Divine, C. and Micoud, M., 1986. Determination of sulbactam in biological fluids by high-performance liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications, 383, pp.218-222. [27] Garcia, M.S., Sanchez-Pedreno, C., Albero, M.I. and Ródenas, V., 1997. Flow-injection spectrophotometric determination of frusemide or sulphathiazole in pharmaceuticals. Journal of pharmaceutical and biomedical analysis, 15(4), pp.453-459. [28] Fischer, F.G. and Bohn, H., 1955. Eine Mikrobestimmung des Ammoniaks, insbesondere in Protein-Hydrolysaten. [29] Reich, E. and Schibli, A., 2007. Highperformance thin-layer chromatography for the analysis of medicinal plants. Thieme. [30] Martin, M. and Guiochon, G., 2005. Effects of high pressure in liquid chromatography. Journal of Chromatography A, 1090(1-2), pp.16-38. [31] Abidi, S.L., 1991. High-performance liquid chromatography of phosphatidic acids and related polar lipids. Journal of Chromatography A, 587(2), pp.193-203.
Copyright
Copyright © 2025 Rohit Pradip Ade , Rutuja Vinayak Bhavar , Sahil Namdev Darade , Anagha Amit Sarvadnya. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66266
Publish Date : 2025-01-03
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

