Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Borehole Water Supply in Enugu Metropolis: Water Quality Comparative Study of Sources from 9th Mile and Emene, Enugu State, Nigeria
Authors: Hyacinth O. Eze, Stanley C. Odinma, Cornelius U. Ozuomba
DOI Link: https://doi.org/10.22214/ijraset.2024.60224
Certificate: View Certificate
Abstract
Water quality study presents status of water for domestic, irrigation, industrial and other uses. Indiscriminate and wasteful water consumption, improper waste disposal practices, and underground contamination have led to deterioration and pollution of water quality. This study was aimed at determining water quality status using parameters in borehole water. Samples from two different towns; Ngwo and Enene, Enugu East. Sampling locations 1(6o28’24.7”N 7o34’49.2”E) and 2(6o27’36”N 7o36’46”E) are situated within Nike East Local Government (precisely Emene) while sample 3(6o25’52.8”N 7o25’10.6”E), 4(6o25’55.3”N 7o24’22.8”E) and 5(6o25’50.4”N 7o26’08.5”E) are within Enugu Ngwo (9thMile Corner). The parameters analyzed include; Electrical conductivity, Turbidity, pH, TDS, TSS, SS, Calcium, Magnesium, and Total Hardness, using laboratory standard methods of analysis. The data provided by this investigation shows that the water samples from Enugu Ngwo only is more acidic outside the recommended level. Other qualities of all the parameters are all measurable for both locations. Based on these findings, recommendations were presented for the purpose of improving water quality and sustainable assess in Enugu Metropolis and beyond.
Introduction
I. INTRODUCTION
It is obvious that the world population cannot be sustained without access to safe water. Therefore, it is important to conjunctly consider both water quality and quantity in water resources management (Xinghui, 2009). The essence of water quality management is usually to minimize the health risks associated with either direct or indirect use of water (Udom, Ushie, & Esu, 2002). Contamination of water has increasingly become an issue of serious environmental concern after years of pollution (Akpoveta, Okoh, & Osakwe, 2011).
The World Health Organization (WHO) recommends that the minimum daily per capita water consumption to be 27 litres/person/day. However, many people manage with far less than 27 liters (Franceys, Pickford, & Reed, 1991). The increasing population pressure and rising demand for food and other services has increased demand for water. This has increased reliance on groundwater resources thereby creating challenges among which are the provisions of adequate quantity and quality of water.
In Nigeria, despite the involvement of the World Bank, African Development Bank, and United Nations Children’s Educational Fund (UNICEF) to improve drinking water quality, access to safe water is still a challenge (Ajayi et al, 2008). There is need to subject drinking water to safety and quality tests regardless of its source. According to Ishaku et al (2011), water supply in rural areas in Nigeria is a public health dilemma since over 70% of the households do not have access to clean drinking water. Gregory and Victor (2018) also confirmed this by reporting the need to examine issues arising from the increase in boreholes dug in Nigeria and concerns regarding groundwater contamination. Boreholes water have become the most common alternatives for getting drinking water for the average Nigerian. However, reports indicated that most of these sources might not meet acceptable water quality for consumption purposes.
Boreholes and wells locally distort the natural flow field and create a path that opens up an additional possibility of heat and mass transfer between rock formations / aquifers, surrounding and atmosphere (Akpoveta, 2011). Indiscriminate waste disposal, poor agricultural practices, septic tanks, pit latrines and graves near boreholes, poor well construction, contribute to borehole water contamination (Egwari & Aboaba, 2002). These account for the presence of coliform bacteria in borehole water.
A. Statement of Problem
The fact that water is not easily accessible to numerous sections of the global population defines the central management problem of borehole water resources. The need to balance the dependence of groundwater utilization against the increasing global pollution threats from industrialization, urbanization and agricultural activities, facilitated this work
The need to access safe water has forced many developing nations to opt for providing safe drinking water from boreholes. There is an increasing understanding that contaminated water consumption is responsible for several health-related disorders such as waterborne infections like diarrhea and typhoid fever. The proliferation of unregulated boreholes in Enugu and its surrounding environment has raised concerns about water quality and suitability for safe drinking and other domestic purposes in line with regulatory standards. Furthermore, the clamor and high demands of borehole water from 9th mile than those from other parts of Enugu State including Emene which also has boreholes calls for serious study on the comparative analysis of the physiochemical parameters of the water samples. It is necessary that the quality of drinking water should be checked at regular time interval, because due to use of contaminated drinking water, human population suffers from varied forms of water borne diseases.
B. Aim and Objectives
The Aim of this research is to compare the quality of borehole water from 9th mile and Emene which supply water to Enugu Metropolis. The objectives of the study are focused on:
- developig an experimental backup on the ideology of the people of Enugu State, “that 9th mile Borehole water is better than the others”;
- examining the level of physico-chemical Parameter and mineral compositions of borehole water collected from two different locations;
- comparing the values obtained from analysis with that of national and international standards with a view to improving the quality.
II. CONCEPTUAL FRAMEWORK AND REVIEW OF THE RELATED LITERATURE
Numerous studies have assessed water quality parameters and accessibility in various rural and urban sectors of the world.
A. Groundwater and Its Quality
Ground water is the water that is found below the surface of the earth, where it occupies all or part of void spaces in geological layers. It is also called subsurface water to distinguish it from surface water (The New Encyclopaedia Britannica 1974; Zumdahl,2020).It is that portion of the atmospheric precipitation mostly rainfall, which has percolated into the earth to form underground deposits called aquifers (water-bearing formations). The quality of water is of vital concern for mankind since it is directly linked with human welfare. According to Rajana (2010), the quality of public health depends to a greater extent the quality of groundwater. Though groundwater quality is believed to be quite good compared to surface water, its quality is the sum of natural: geology of the environment and anthropogenic influences (withdrawal, land use change, and solid waste dumping). Water quality parameters reflect the level of contamination in water resources and show whether water is suitable for human consumption. Contaminated water is unacceptable due to health effects, poor taste and aesthetic value to consumers.
B. Parameters for Defining Water Quality
The usefulness of water for a particular purpose is determined by the water quality, which is determined by the solutes and gases dissolved in the water as well as the matter suspended in and floating on the water. It is a consequence of the natural physical and chemical state of the water as well as any alterations that may have occurred as a consequence of human activity. If human activity alters the natural water quality so that it is no longer fit for purpose which it had previously been suited, the water is said to be polluted or contaminated. In any case, water quality is usually affected directly or indirectly by human activities making the harmful for living plants and animals".
C. Physical properties/characteristic
- Electrical conductivity: this is a quantitative measure of the ability of water to conduct electric current. It can also be defined as a numerical expression of the ability of an aqueous solution to carry an electric current (Lind, 1959). Electrical conductivity is influenced by the presence of dissolved salts such as sodium chloride and potassium chloride which produce ion that migrate in solution and then generate electric current.
Electrical conductivity is also a measure of the total dissolved solid (TDS) or salinity (Lind, 1959). Conductivity shows significant correlation with ten parameters such as temperature, pH value, alkalinity, total hardness, calcium, total solids, total dissolved solids, chemical oxygen demand, chloride, and iron concentration of water.
2. Turbidity: is a measure of the loss of transparency of a solution. The presence of colloidal solid gives water a cloudy appearance which reduces its transparency.
3. Taste and odour: when impurities are dissolved in water, the taste and odour become objectionable.
4. Solids (Total Dissolved and Suspended Solids) Total Suspended Solid (TSS): These are discrete particles that can be measured by filtering the sample through appropriate filters. The magnitude of these solid depends on the type of filter (paper or sintered glass used), the pore size, the physical nature and the size of the particles (ASTM, 2004). Increase in suspended solid in water is proportional to the increase in the extent of pollution and also account for odour and colour (Golterman, 1978). The materials deposited on the filter are the principal factors affecting separation of suspended solid from dissolved solid. Total Dissolved Solid (TDS): is defined as the concentration of all dissolved minerals in the water. Total dissolved solids are due to soluble materials. These refer to the portion of total solid that pass through the filter and is express in Mg/L. High water with high dissolved solid is generally of inferior palatability and may induce an unfavourable physiological reaction in the transient consumer. High concentration of dissolved solid in water is also responsible for hardness, turbidity, odour, taste, colour and alkalinity (ASTM, 2004). The maximum permissible concentration of TDS is 500mg/L in potable water. Poor chemical quality of water is a health risk in the long term for consumers. Urban waste waters are often high in nutrients concentrations (macronutrients Na, Ca, P, K, Mg and micronutrients Fe, Zn, Cu,) and other chemicals which can stress the bacterial populations, in rainy seasons they are washed to the groundwater by infiltration (Thomas, 1995). The chemical composition of groundwater may be altered by the precipitation of ions from solution to form insoluble compounds.
- Colour: Good water should be transparent and clear (colourless). The colour of water is expressed in Hazen units which correspond to the colouration of a series of platinum/cobalt.
- Temperature: the temperature of water is not the main issue when considering it as physical parameter, but its effect on other properties e.g. changing solubility of gases.
D. Chemical properties/characteristics
- pH is a measure of the hydrogen ion (H+ ) available in water. The acidity of groundwater is due to the presence of organic acids in the soil as well as those of atmospheric origin infiltrated to the water (Chapman & Kimstach, 1996). Acid rain contains dissolved Carbon dioxide (CO2), Nitrogen dioxide (NO2) or Sulphur dioxide (SO2) often yields an elevated Hydrogen ion (H+) ion concentration and Carbonic acid (HCO) and may cause serious threat to groundwater pH. The pH of rainwater is about 5.7. Increase in acidity is also attributed to the oxidation of reduced Sulphur compounds in the soils of the areas (Efe et al, 2005). The pH affects the solubility and toxicity of metals by influencing chemical kinetics of important constituents. Other acids such as HNO3, HNO2 and humic acid are formed as a consequence of the decomposition of organic matter and sulphuric acid is produced when minerals such as pyrite (FeS2) breakdown. High pH levels make water to become less corrosive.
- Alkalinity is a water characteristic that shows the capacity of water to neutralize acids by accepting Hydrogen ions (H+) and preventing sudden changes in the acidity levels of water. Alkalinity is due to the presence of two forms of the Carbonate anions (HCO3-), (CO32-) and (OH-) that act as buffer system. Borates, phosphates, silicates and other bases also contribute to alkalinity if present in groundwater. Inorganic ligands (anions) form complexes with metals (cations), this removes free divalent toxic metal ions such as Cd2+, Cu2+, Pb2+, Zn2+ or methyl-metal complexes. Metal complexes are not biologically available and hence not toxic. Alkalinity is an important property when determining the suitability of water for other uses such as irrigation, or mixing with pesticides and when treating contaminated water. Alkalinity is measured in CaCO3 mg/L. According to Fakoyode (2005), pH that is near to neutral (pH 7) is indicative of unpolluted water. Carbon dioxide (CO2) readily dissolves in water as illustrated in equation 1. The dissolved CO2 (aq) reacts with water molecules to form Carbonic acid (H2CO3) as shown by equation 2 and Carbonic acid is very unstable and quickly dissociates into H+ and a Bicarbonate ion (HCO3- ) as demonstrated in equation 3. At pH 6.3, the amount of CO2 dissolved in water equals the amount of bicarbonate ion (HCO3-). Dissolved carbon dioxide is dominant when pH is<6.3. At higher pH, basic water, HCO3- dissociates to yield H+ and a Carbonate ion (CO32-).
- Nitrate: Nitrate contamination of groundwater results from leaching of fertilizer, septic tank leachate, unsewered sanitation, pit latrines, animal waste or human waste mineralization of decomposing or oxidation of decaying matter by soil micro-organisms.
Unutilized urea leached to groundwater for micro-organisms to degrade is also another source of groundwater nitrate (Singh, 2012). Nitrate can readily be transported beneath the soil zone because it is relatively soluble and not prone to ion exchange (Stumm and Morgan, 1996). Nitrate can be endogenously reduced to nitrite, which can then undergo nitrosation reaction in the stomach with amines to form a variety of Nnitroso compounds (NOC). These compounds are carcinogens, thereby causing health hazards like impairing the ability of the blood to carry oxygen (Blue-baby syndrome or infantile methemoglobinemia), gastrointestinal cancer, Alzheimer disease, vascular dementia, adsorptive secretive functional disorders of the intestinal mucosa, multiple sclerosis, Non-Hodgkin’s lymphoma and hypertrophy of thyroid (Suthra, 2009). Nitrate contamination can be treated by technologies such as ion exchange; denitrification and reverse osmosis or anaerobic reduction in the subsurface which can limit Nitrate contamination of groundwater (Kapoor & Viraraghavan, 1997).
4. Calcium carbonate: Hardness refers to the ability of water to form suds with soap. Hard water leaves a ring in the bathtub, forms soap curds in clothing, and builds up scale in boilers and kettles (Wittmann et al., 1998). Hardness is divided into two: Carbonate hardness Ca(HCO3)2 and nonCarbonated hardness Mg(HCO3)2. Non hardness is due to presence of salts such as Calcium Chloride (CaCl2), Magnesium Sulphate (MgSO4) and Magnesium Chloride (MgCl2). Any hardness greater than the alkalinity represents non-Carbonate hardness is measured as Calcium Carbonate mg/L. Hardness is classified as soft, moderately hard, hard and very hard. Areas with limestone formations have a higher hardness and alkalinity due to the dissolution of Bicarbonates and Carbonates. Calcium in groundwater is derived from Calcite, Aragonite, Dolomite, Anhydrite and Gypsum. In igneous and metamorphic rocks calcium is supplied by the feldspars, pyroxenes and amphiboles and the less common minerals such as Apatite and Wollastonite. Water hardness is an important component of water because it has a bearing on the portability of water. Water can be classified based on its hardness according.
5. Iron: Iron is not toxic, but imparts objectionable taste to water and may leave brown stains on porcelain and in clothing. Objectionable taste is due to reduced form (Fe2+ and HS), on exposure to air, water becomes reddish brown due to Ferric Hydroxide and prolonged consumption of such water may lead to liver disease (Ranjana, 2010). Largest contributors of iron in groundwater are minerals contained within the underlying bedrock, soil and sand, the most common is Ferrous Iron and borehole, limestone, shale and coal which often contain the Iron rich mineral Pyrite, acidic rain also releases Iron into groundwater. Iron content increases with depth. An aquifer in which groundwater is in a mildly oxidized state and a near neutral pH, the most likely Iron is Fe3+ and is tied up in solid phases. At a given temperature changing from their oxidized form / giving up of electrons (Fe3+ and SO2- ) to the reduced (accepting electrons) form requires a decrease in redox potential (dissolved oxygen) or a decrease in pH. Nitrate to Nitrogen gas, Fe3+ (insoluble) to Fe2+ (soluble), Sulphate to Hydrogen Sulphide and at very low redox potential, Methane formation occurs. Reduction / treatment of iron can be achieved by using a water softener, Potassium Permanganate or green sand filters and aeration (addition of oxygen to water) all aid in precipitation of Iron. Salts may be concentrated in the groundwater as result of evaporation and transpiration. This depends on vegetative cover, warmth, soil type, and climate (Soveri, 1985).
6. Manganese (Mn): It is an essential trace element with an estimated daily nutritional requirement of 30-50 mg/kg of body weight, it is one of more abundant metals in the earth's crust and usually occurs together with Iron. Dissolved manganese concentrations in ground and surface waters that are poor in oxygen can reach several milligrams per litre. On exposure to oxygen, manganese can form insoluble oxides that may result in undesirable deposits and colour problems in distribution systems. The presence of it in drinking water like that of iron, at levels exceeding 0.1 mg/l, in water supplies stains sanitary ware and laundry and causes an undesirable taste in beverages, at a concentration of 0.02 mg/l it will often form a coating on pipes. It is highly insoluble in natural waters, the solubility increases with increasing acid. The limit of detection by flame atomic absorption is 3.0 mg/l, therefore concentrations above drinking water limit can be detected by this technique.
7. Potassium (K): Potassium (K+) is an alkaline metals closely related to sodium, it is slightly less common than sodium in igneous rocks but more abundant in all sedimentary rocks. The potassium content of natural water is usually less than that of sodium, it seldom occurs in high concentration in natural water. Concentrations of it more than a few tens of milligrams per litre are decidedly unusual except in water having high dissolved solids concentration or in water from hot springs. Potassium is not a major component in public or industrial water supplies.
8. Sodium (Na): It is the most abundant member of the alkali metal group of the periodic table. The sodium ion is ubiquitous in water, most water supplies contain less than 20 mg of sodium per litre. Although concentrations of it in potable water are typically less than 20 mg but in some courtiers levels can exceed 250 mg/I. Sodium salts are generally highly soluble in water and are leached from the terrestrial environment to ground water and surface water, also sodium salts are found in virtually all food and drinking water.
9. Zinc (Zn): It occurs in small amounts in almost all igneous rocks. It is found in virtually all food and potable water in the form of salts or organic complexes. Although levels of zinc in surface and ground water normally do not exceed 0.01 and 0.05 µg/L respectively, concentrations in tap water can be much higher as a result of dissolution of it from pipes. Drinking water usually makes a negligible contribution to zinc intake unless high concentrations of it occur as a result of the corrosion of piping and fittings. Drinking water containing zinc al levels above 3 mg/L may not be acceptable to consumers.
Some Toxic elements in the water:
10. Cadmium (Cd): Cadmium is virtually absent from the human body at birth but it accumulates progressively with age. The kidney is the main target organ for cadmium toxicity. Cadmium concentrations unpolluted natural waters are usually below 1µg/l. contamination in drinking water may also be caused by impurities in the zinc of galvanized pipes and solders in fittings, water heaters, water coolers and taps. Although levels in drinking water are usually less than 1µg/l.
11. Chromium (Cr): Chromium is toxic to animals particularly in the hexavalent state although less so to plants. It is widely distributed in the earth's crust. Concentrations or chromium in natural waters that have not been affected by waste disposal are commonly less than 10 µg/L. Total chromium concentrations in drinking water are usually less than 2 µg/L although concentrations as high as 120 µg/L have been reported. Food appears to be the major source of intake.
12. Lead (Pb): Lead is the commonest of the heavy elements, accounting for 13 mg/kg of the earth's crust. It is present in tap water to some extent as a result of its dissolution from natural sources but primarily from household plumbing systems containing lead in pipes solder, fittings or the service connections to homes. The level of lead in drinking water may be reduced by corrosion-control measures such as the addition of lime and the adjustment of the pH in the distribution system.
13. Nickel (Ni): The concentration of nickel in drinking water is normally less than 0.02 mg/L, although levels up to several hundred micro grams per litre in ground water and drinking water have been reported. Nickel concentrations in drinking water may be increased if raw waters are polluted by natural or industrial nickel deposits in the ground. The average daily dietary intake is normally 0.1-0.3 mg of nickel. It is release from taps and fittings may contribute up to 1 mg/L.
- Micro-biological parameters - Total and Faecal coliforms: According to Bodoczi (2010), the sanitary quality of water is appreciated by the presence or absence of pathogenic micro-organisms indicated by presence of coliforms. There is practically no geological environment at or near the earth’s surface where pH will not support some form of organic life, also at this depth water pressures are not high enough to deter microbial activity (Chapman, 1996). Pathogenic bacteria can survive long underground and may have a life span of about 4 years. Coliform group of bacteria are a large group of disease causing bacteria that inhabit intestine of man and animals (Sirila, Maxwell, Nacarre-Sitchler, & McCray, 2012). Faecal coliform bacteria are bacteria found in faeces, they are subset of a larger group of organisms known as coliform bacteria which are facultative anaerobes that can survive in the absence of oxygen, gram negative, non-spore forming, rod-shaped bacteria that ferment lactose, producing gas and acid at about high temperatures of 35oC. Human waste contaminant in water causes water borne diseases such as diarrhea, typhoid, hepatitis and flu-like symptoms such as nausea, vomiting, fever (FAO, 1995). High coliform counts in water samples are an indication of poor sanitary conditions in the community. According to Adekunle and co-worker, (2007) and inadequate and unhygienic handling of solid wastes in the rural and urban areas leads to high concentrations of microbial organisms. In 2006, the Environmental Protection Agency (EPA) published the ground water rule in the United States to keep microbial pathogens out of public water sources to reduce disease incidence associated with disease causing micro-organisms (EPA, 2012).
- Leaching of Pollutants into Groundwater - Leachate contains dissolved organic substances, chemically reduced inorganic substances like Ammonia, Iron, and Manganese which vary according to the hydrology of the site and the chemical and physical conditions within the site The migration of contaminants is controlled by advection in the fracture, exchange between the fracture and the matrix, sorption and molecular diffusion in the low permeability matrix, organic content, saturation level of groundwater, pH, grain size porous matrix, iso-electric point of virus, colloids and bacteria in groundwater aquifers impact contaminant migration rates by either facilitation if they have a smaller retardation factor (Bekhit, El-Kordy, Hassan, 2009). The aquifer is eventually recharged by the influent seepage of surface water, so that some proportion of the pumpage from the borehole is now obtained from the surface source (Christiansen et al., 2008).
- Selection Borehole Sites - Location of boreholes far from any source of potential pollution avoids water contamination. Assessment of the type and loads of contaminants transported from landfill site to the adjacent aquifer and the extent of leachate plumes within the groundwater is used for site investigation and borehole positioning based on geophysical measurements and positioning based on the Bayesian expert system for flow field modeling (Abbaspour, 2000).
- Purification of Groundwater by Soil - As water passes through fine grained porous media such as soil and rock, impurities are removed by filtration. Some substances react with minerals in the soil/rock and some are oxidized and precipitated from solution. Adsorption may also occur in argillaceous or organic material (Adekunle et al, 2007). According to Vladimir (2003), the capacity to retain, adsorb, detoxify and immobilize micro pollutants such as nutrients, organic chemicals and metals is not constant. Land use can impact soil retention potential for micro pollutants. High organic matter content in soil causes a high retention potential for micro-pollutants (Vegter, 1995). A higher organic matter content causes a high retention potential for micro-pollutants.
- Need for Groundwater Exploitation - Boreholes and wells are groundwater types that form an integral part of water supply systems in rural and urban areas especially in Africa, and therefore are indispensable because of inadequate public water supply systems. Over one billion people lack access to clean safe water worldwide, up to 300 million rural people in Sub Sahara Africa have no access to safe water supplies and this is on the rise (NAS, 2009). There is an increasing demand for large amounts of water as health and sanitation improve. Without safe water near households, the health and livelihood of families can be severely affected (United Nations, 2010). To solve such issues relating to water borne diseases, boreholes can provide safe and convenient water supply since it is evenly distributed, affordable with quiet good quality and not affected by seasonal changes hence its sustainable (Adekunle et al., 2007). The only realistic option for meeting rural water demands is through groundwater exploitation. A large population of the world especially in sub-Sahara Africa depends on groundwater as their main source of domestic water, this is because it is accessible anywhere, less capital intensive to develop and maintain and is less susceptible to pollution and seasonal fluctuation, naturally has good quality. Water resources availability is of significance to regional social-economic development and is seen as a limiting factor in human development. Groundwater plays a vital role in the development of arid and semi-arid zones (Ranjana, 2010) and its development especially borehole water in Africa is seen as more amenable to poverty targeting than surface water. A greater proportion of household income may need to be spent on water delivered from private sources, such as tankers to supplement lack of water locally.
E. Water Security
Water security mapping can help identify vulnerable areas and changes to monitoring systems can ensure early detection of pollution problems (Akpoveta, 2011). Water security includes efforts in reduction of effort and time required to collect water, reduction in workload of women, improvement of availability of water, increasing the quantity of water consumed per capita per day and increasing production activities such as crop washing especially small scale gardening as social conditions which could be improved by developing community water supply. Increasing the coverage of groundwater based rural water supplies can significantly increase the reliance of rural communities to climate variability.
F. Borehole Water Availability and Accessibility
About 70% of the earth’s surface is covered by water, of all the water on earth approximately 3% is fresh water and less than 1% of the world’s fresh water is accessible for human use. Water shortages and difficulties in accessing water affect domestic and productive livelihoods of communities. Proximity to water resources increases per capita consumption and encourages water use for vegetation and fruit production. Therefore, there is need to increase reliability of sources by improving water coverage and prioritizing vulnerable areas. The world is facing a water crisis and it is indispensable that there is not enough clean water available to meet today’s populations’ needs (WWF, 2000). Access to adequate supplies of good quality drinking water continues to be limited among many rural and peri- urban communities of Africa, despite several years of water improvement programmes (Mireilleet al., 2011). Climate change alters hydrological cycle ranging from evaporation, precipitation, runoff, groundwater to re-charge, decreasing seasonal rainfall trends (Akpodiogaga and Odjugo, 2010).
Water Quality Control: The use of water for industrial, agricultural and domestic, purposes causes deterioration in quality. This polluted water is harmful to the environment if not treated before its release back into use. Water quality control includes the removal of:
- Excessive colour, test and odour;
- Objectionable dissolved matter;
- Aggressive constituents; and
- Bacterial indication of faecal pollutants. However, different water samples contain different level of contaminant. Therefore, parameters defining the quality are diverse and they vary with the corresponding water samples or use to which the water is put.
- Empirical Review: Studies on the quality of water consumed in rural communities in Nigeria by scholars like (Adekunle et al, 2007; Essien, and Bassey, 2012) in Igbora and Uyo, Southern Nigeria and found that the quality of water from hand-dug wells were polluted by human activities and therefore, unsuitable for human consumption. Similarly, the study of Adediji and Ajibade (2005) confirmed the unsuitability of well water for human consumption in Ede area of southwest Nigeria. They identified human activities as likely sources of pollutants to the groundwater. Maxwell et al. (2010) examined the spatial distribution of iron across rural communities of Benue State and attributed the variations in iron concentration to the geology of the study area. Omoboriowo et al. (2012) observed that the groundwater in Arochukwu area of Afikpo Basin were generally soft, free from saltwater intrusion and low with iron constituents. Awoyemi et al. (2014) established that the groundwater problems in Majidun-Ilaje rural community of Ikorodu west LGA of Lagos State was due to the pollution of groundwater by pollutants from natural sources. Weli and Ogbonna (2015) examined the relationship between water quality parameters and water borne diseases. They identified the major contaminants in the well water samples and posited that the prevalent water borne diseases in the area may probably be due to the consumption of the degraded well water. Uzoije et al, (2014) ascertain the chemical constituents of deep and shallow aquifer waters in the rural areas of Nsukka and the contributions of household, industrial and agricultural pollutants to its impaired quality. Similarly, the water quality status of shallow and deep aquifers wells from the rural communities of Nsukka and discovered that while the aquifers are highly polluted by iron, the shallow aquifers are polluted by human activities. The influence of environmental factors on well and borehole water quality in rural communities in Nigeria was investigated (Obeta & Mamah, 2018), which characterized the pollutants, determined the influence of environmental factors on the water quality and highlighted the health implications of the findings. The analysis showed borehole samples exhibit higher concentration of natural pollutants while well samples exhibit higher concentration of anthropogenic pollutants.
- Literature Gap: It is unfortunate that borehole sinking and use is not regulated by state agencies and the level of risks to which users are exposed remain unascertained. The also state is yet to come out with policies to safeguard the health of the well water users and/or tackle the challenges of well water contamination in the area. To intervene in this direction, there is the need for an in-depth understanding of the underground water quality status and of the natural and anthropogenic factors influencing the water chemistry of the areas which is currently lacking. This is necessary both for planning purposes and to verify the concerns of the people about the deteriorating quality of water available for their consumption.
III. THE STUDY AREAS, MATERIALS AND METHODS
Enugu State is in the South East Geo-political Zone of Nigeria. It is located at 6o30' North of Equator, and 7o30' East of Latitude. It is plus one hour (+1hr) GMT on the World Time Zone. It shares border with the following states: Abia and Imo to the south; Ebonyi to the east, Benue to the north-east, Kogi to the north-west and Anambra State to the west. It covers an area of 7,161 km2 (2,765sq mi), and ranks 29th out of the 36 States of Nigeria in terms of land area. Enugu State has a good climatic condition all the year round. The hottest month is February with about 87.16 oF (30.64 oC), while the lowest temperature is recorded in November/December, reaching about 60.54 oF (15.86 oC). Lowest rainfall of about 0.16 cubic centimeters (0.0098 cu in) is recorded in
February, while the highest rainfall is recorded in July at about 35.7 cubic centimeters (2.18 cu in).
It has an estimated population of 3,267.837, (1,596,042-males and 1,671,795- females) according to (NPC, 2006 Census).
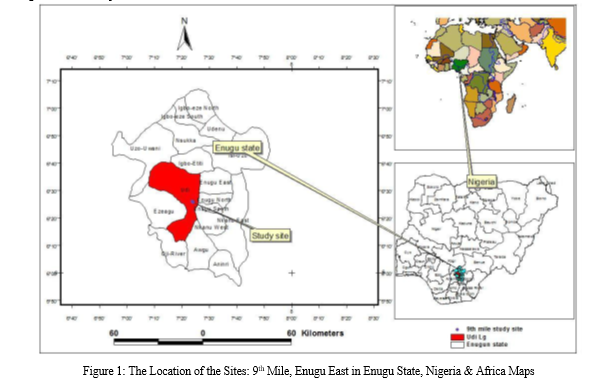
Enugu Metropolis is made up of three Local Government Areas namely, Enugu North, Enugu South and Enugu East; while Emene is originally regarded as a satellite community to Enugu City and one of the communities in Enugu East LGA. 9th Mile on the other hand is situated in Udi LGA of Enugu State along 7o 24’20”E as provided in figure 1.It is located approximately 10.4 Km away from Enugu Metropolis. There are limited number of boreholes in Emene. Water has been an important issue in this part of the country especially during the dry season; meanwhile there has been a long term notion that water from Ngwo, particularly 9th Mile is considered the best from the general public which necessitated this study.
Sampling Locations: The study covers two sampling area, namely 9th Mile and Emene, with five sample points. These five sampling locations are: 1. (6o28’24.7”N 7o34’49.2”E) and 2. (6o27’36”N 7o36’46”E) situated within Nike, Enugu East Local Government Area (precisely Emene) while sample 3.
(6o25’52.8”N 7o25’10.6”E), 4. (6o25’55.3”N 7o24’22.8”E) and 5. (6o25’50.4”N 7o26’08.5”E) are within Ngwo (9th Mile Corner). See Figure 1 for the map details.
Source: Enugu State NEWMAP Final ESMP Report for 9th Mile Corner Gully Erosion Site, 2014
The parameters analyzed include; Electrical conductivity, Turbidity, pH, TDS, TSS, SS, Calcium, Magnesium, and Total Hardness, using laboratory standard methods of analysis. The laboratory where these analyses were conducted is Power Tech Analysis and Scientific Research Laboratory; Shop 4A Institute of Management and Technology (IMT) Campus 3 Enugu, Enugu State.
IV. RESULTS AND DISCUSSION
Sampling locations 1(6o28’24.7”N 7o34’49.2”E) and 2(6o27’36”N 7o36’46”E) are situated within Nike East Local Government (precisely Emene) while sample 3(6o25’52.8”N 7o25’10.6”E), 4(6o25’55.3”N 7o24’22.8”E) and 5(6o25’50.4”N 7o26’08.5”E) are within Enugu Ngwo (9th Mile). The results of the analyses are shown in the table 1.
Table 1: Result of the Analysis of the Samples of the Sites’ Water.
|
S/N |
PARAMETERS |
UNIT |
Sample 1 |
Sample 2 |
Sample 3 |
Sample 4 |
Sample 5 |
NESREA / SON STANDARDS LIMITS |
WHO STANDARD LIMIT |
METHODOLOGY |
|
1 |
Appearance |
|
transperent |
transperent |
transperent |
transperent |
transperent |
colourless |
|
Optical view |
|
2 |
Temperature |
oC |
28 |
28 |
28 |
28 |
28 |
40oc |
30.5 |
Thermometer |
|
3 |
Salinity |
|
7.38 |
7.58 |
6.21 |
5.86 |
9.43 |
|
|
|
|
4 |
pH |
|
6.16 |
7.24 |
5.98 |
5.56 |
5.26 |
6.5-8.8;6-9 |
6.5-8.5 |
pH meter |
|
5 |
Electric conductivity |
µs/m |
180 |
149 |
46 |
48 |
190 |
|
|
|
|
6 |
Turbidity |
NTu |
3.98 |
4.11 |
4.30 |
4.31 |
4.42 |
5 |
5 |
|
|
7 |
Total Suspended Solids |
Mg/l |
0.013 |
0.007 |
0.002 |
0.010 |
0.004 |
25 |
|
Photometric |
|
8 |
Total Solids |
Mg/l |
90.013 |
74.507 |
23.002 |
24.01 |
95.004 |
N S |
|
Additional of TDS and TSS |
|
9 |
Total Dissolved Solids |
Mg/l |
90 |
74.5 |
23 |
24 |
95 |
500 |
|
Digital DO meter |
|
10 |
Chloride |
Mg/dl |
18 |
20 |
16 |
20 |
26.5 |
250 |
250mg/l |
APHA |
|
11 |
Sulfate |
Mg/dl |
49.38 |
76.018 |
16.46 |
20.575 |
90.53 |
100 |
500mg/l |
APHA |
|
12 |
Phosphate |
Mg/dl |
17.614 |
27.273 |
10.795 |
44.034 |
39.205 |
|
|
APHA |
|
13 |
Nitrate |
Mg/l |
2.191 |
7.121 |
1.711 |
15.884 |
4.304 |
50 |
50mg/l |
APHA |
|
14 |
Total Hardness |
Mg/l |
25 |
16 |
20 |
15 |
30 |
|
500 |
APHA |
|
15 |
Potassium |
Mg/l |
2.5043±0.0049 |
1.360±0.0006 |
0.3360±0.0023 |
1.0418±0.0043 |
2.4995±0.0000 |
|
|
AAS |
|
16 |
Calcium |
Mg/l |
6.25±0.0004 |
ND |
ND |
ND |
0.9259±0.0000 |
|
|
AAS |
|
17 |
Sodium |
Mg/l |
4.1636±0.0134 |
7.4120±0.0568 |
1.3964±0.0070 |
1.3119±0.0341 |
4.1610±0.0356 |
|
200mg/l |
AAS |
|
18 |
Iron |
Mg/l |
ND |
ND |
ND |
ND |
ND |
0.3 |
|
AAS |
|
19 |
Magnesium |
Mg/l |
1.9795±0.00204 |
0.2778±0.0046 |
0.3360±0.0023 |
0.1360±0.0062 |
0.8383±0.0021 |
|
50 |
AAS |
Source: Researchers’ Field Survey Data Analysis from the Water Samples


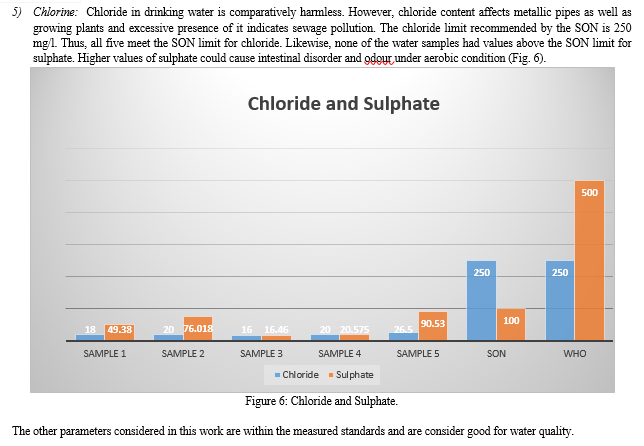
Conclusion
Conclusions have been drawn based on the objectives of the study attained and relevant recommendations provided. 1) Conclusion: Most of the parameters analyzed (90%) for water from the boreholes in Enugu East and Ngwo area were within the acceptable range as recommended by SON and WHO for drinking water. None of the samples analyzed was found to contain any residual chlorine. Therefore, the water from boreholes in the both study areas is suitable for drinking, bathing, recreation, irrigation and industrial uses. It was also observed from the analysis that all the samples from Ngwo area fall outside the recommended level for pH values. In in other words, contrary to the common opinions, water from 9th Mile is more acidic than those from Enugu East. Thus, it can be concluded that the borehole water is within the safe limits and fit for domestic consumption. 2) Recommendations: Based on the findings concluded above, the following recommendation are made as follows: a) Government policies should utilize the findings of this research to expand the access to quality water, regulation of borehole locations as well as making the process flexible and liberal for developers and investors in water exploration and supply; b) An enabling environment should be created for better public/private partnership in funding of borehole projects to be incorporated into planning and development of Enugu Metropolis and beyond; c) Emene borehole option should serve as a reliable alternative towards reducing highly competitive and costly supply of water from 9th Mile Corner; d) There is need for awareness creation about the findings as a means of added opportunity for expanding water security in Enugu through Emene supply; and e) Further studies are recommended on the areas of availability and complexity associated with exploring Emene borehole water as compared with that of 9th Mile.
References
[1] Abbaspour et al., (2000). A contaminated site investigation: comparison of information gained from geophysical measurements & hydro geological modeling. 40- 2000. 365-380. [2] Adediji, A. & L.T. Ajibade, (2005). Quality of well water in Ede Area, Southwestern Nigeria. J. Hum. Ecol., 17: 223-228 [3] Adekunle, I.M., Adetunji M.T., Gbadebo A.M. & Banjoko O.B., (2007). Assessment of groundwater quality in a typical rural settlement in Southwest Nigeria. Int. J. Environ. Public Health, 4: 307-318 [4] Ajayi, A., Sridhar, M. K., Adekunle, L., & Oluwande, P. (2008). Quality of package waters solid in Ibadan, Nigeria. African Journal of Biomedical Research, vol.11 251-258. [5] Akpodiogaga P, Odjugo O (2010). General Overview of Climate change Impacts in Nigeria. J Hum Ecol. 29(1): 47-55 [6] Akpoveta O.V., Okoh B.E., & Osakwe S.A. (2011). Quality Assessment of Borehole Water used in the Vicinities of Benin, Edo State & Agbor, Delta State of Nigeria. Current (2013). Health and environmental components of sachet water consumption & trade-in Aba & Port Harcourt, Nigeria. Journal of Chemical Engineering Materials Science, Vol. 4(2). pp. 13.22 [7] Bekhit H.M., El-Kordy M.A., Hassan A.E., (2009). Contaminant transport in groundwater in the presence of colloids & bacteria: Modder development & verification. Irrigation and Hydraulics Department, Cairo University. Journal of Contaminant Hydrology 108 152-167 [8] Bodoczi, A. (2010). The Seasonal Quantitative Distribution of Coliform Germs in the Arie? River (Romania) Water Affected By Pollution [9] Christiansen, C.M., Riis, C., Christensen, S.B., Broholm, M.M., Christensen, A.G., Klint, K.E.S., Wood, J.S.A., Bauer-Gottwein, P., Bjerg, P.L., (2008). Characterization & quantification of pneumatic fracturing effects at a clay till site. Environmental Science & Technology. 42 (2), 570– 576. ) [10] Chapman, D. &. Kimstach, V (1996). Selection of Water Quality Variables. Water Quality Assessments: A Guide to the use of Biota, Sediments & Water in Environmental Monitoring. Chapman edition, 2ndEdn. E & Spon, London FN, 595. [11] Efe S.I., Ogban F.E., HorsfallM.Jnr., & Akporhonor E.E. (2005). Seasonal variations of physico-chemical characteristics in water resources Quality in western Niger Delta Region Nigeria J. Appl. Sci. Environ. Mgt. 9(1): 191-195. [12] Essien, O.E. & E.D. Bassey, (2012). Spatial variation of borehole water quality with depth in Uyo Municipality, Nigeria. Int. J. Environ. Sci. Manage. Eng. Res., 1: 1-9. [13] Egwari L., & Aboaba, O.O. (2002). Bacteriological quality of domestic waters. Rev. Saude Publica, 36(4): 513-520. [14] FAO, (1995). Land & Water Development. Technical Paper Series, No. 1. Rome, Italy. [15] Fakayode, S.O. (2005). Impact Assessment of industrial Effluent on Water Quality of the Receiving Alaro River in Ibadan Nigeria. AjeanRagee 10:1-13 [16] Franceys, R., Pickford, J & Reed, R. (1991), A guide to the Developing & Managing Community Water Supplies of on-site Sanitation, Geneva: World Health Organization. [17] Gregory, E & Victor, N. (2018). Impact of proliferation of borehole development projects on ground water quality in Abia State, Nigeria. ISSN: 0974-3987. International Journal of Biosciences & technology. Volume 11, Issue [18] Hillel, R. & Rabideau, A.J. (2000). Approximate analysis of the containment of contaminated sites prior to remediation, Water science and Technology, 42 (1-2), 319-324. [19] Ishaku, H., T., Majid, M. R., Ajayi, A., &Haruna, A. (2011). A water supply dilemma in Nigeria rural communities, looking towards the sky for an answer. Journal of Water Resources & Protection 3, 598-606. [20] Kapoor, A., Viraraghavan, T. (1997). Nitrate removal from drinking water review. J. Environ. Eng. 123 - 4.371–380. [21] Lind, J.E. (1959); Water Pollution, Clarendon Press, Oxford, p. 9. [22] Majuru, B., Mokoena M.M. Jagal., P & P.R. Hunter, 2011. Health impact of small-community water supply reliability. Int. J. Hyg. Environ. Health, 214: 162-166. [23] Maxwell, O., I. Mile & M.C. Obeta, 2010. Seasonal variation in nitrate levels in hand dug wells in Makurdi Metropolis. Pak. J. Nutr., 9: 539-542. [24] National Archives of Scotland (NAS), (2009). Sustainable Development & Environmental Policy Document. [25] Omoboriowo, A.O., Chiaghanam O.I., SoronnadiOnoniwu G.C.,. Acra E.J & K.O. Okengwu et al., 2012. Appraisal of the groundwater quality in Arochukwu Area, Afikpo Basin, Nigeria. Int. J. Sci. Technol., 2: 788-793. [26] Obeta Michael Chukwuma & Mamah Kingsley Ifeanyichukwu (2018). Influence of Environmental Factors on the Physico-Chemical and Bacteriological Quality of Well and Borehole Water in Rural Communities of Udenu LGA of Enugu State, Nigeria. Pak. J. Nutr., 17 (11): 596-608. [27] Rajana A. (2010). Physico-Chemical Analysis of some Groundwater Samples of Kotputli Town Jaipur, Rajasthan India. Vol. 1, No.2, 111-113. [28] Sirila E.R., Maxwell, R.M., Nacarre-Sitchler, A.K. & McCray, J.E. (2010). A quantitative methodology to assess the risks to human health from carbon dioxide leakage into ground water. Advwater resource. [29] Suthra, .S. Bishnoi, P., Singh, S. Mutiyar, P.K., Nema, A.K. & Patil, N.S. (2009). Nitrate contamination in groundwater of some rural areas of Rajasthan, Department of Civil engineering, Indian Institute of Technology New Delhi India. [30] Singh, B., Khurana S.C., Manish K., Tadav N., & Yadav R. (2012). Seasonal Variation of Coliforms and Nitrate in Groundwater Quality in Kanpur Metro. India. International Journal of Research in Chemistry and Environment Vol. 2 Issue 2 April (207-209). [31] Stumm, W. & Morgan, J.J. (1996). Aquatic Chemistry, 3rd edition. Wiley-Interscience, New York, 1022 pp. [32] \"The New Encyclopaedia Britannica\" l5th Ed., Macropaedia, Vol. 8, Encyclopaedia Britannica, U.S.A., (1974), p. 432. [33] Udom, G.J. Ushie, F.A. & Esu, E.O. (2002).A geochemical survey of groundwater in Khana and Gokana local government area of Rivers State, Nigeria. J. Applied Sci. Environ. Management. 6: 53-59. [34] United Nations, (2010). Water Global Annual Assessment of Sanitation & Drinking- water. [35] Vegter, J. (1995). Soil protection in the Netherlands, in Heavy Metals: Problems and Solutions, (W. Salomons, P.Mader, and U. Fo”rstner, eds.), Springer-Verlag, Berlin. [36] Weli, V.E. & V.A. Ogbonna, 2015. An analysis of well water quality & the incidence of water borne diseases in Emohua communities, Rivers state, Nigeria. Int. J. Environ. Pollut. Res., 3: 32-41. [37] Wittmann E., P. Cote, Medici, C. Leech J. & Turner, A.G (1998).Treatment of hard borehole water containing low levels of Pesticide by Nano filtration. Desalination 119: 347-352. [38] Xinghui, X. Zhifeng Y. & Yuxiang W. (2009). Incorporating Eco-environmental Water Requirements in Integrated Evaluation of Water Quality & Quantity- A case study for the Yellow River. Water Resour Manage 23:1067-1079. [39] Zumdahl, S. S. (2020). Water. Encyclopedia Britannica. Retrieved from https://www.britannica.com/science/water
Copyright
Copyright © 2024 Hyacinth O. Eze, Stanley C. Odinma, Cornelius U. Ozuomba. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET60224
Publish Date : 2024-04-12
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

